Anti-Xa Level Monitoring for Treatment Dose Enoxaparin in Liver/Intestine Transplant Recipients
Jackson Memorial Hospital, Miami, FL
Meeting: 2019 American Transplant Congress
Abstract number: D216
Keywords: Anticoagulation, Intestinal transplantation, Liver transplantation
Session Information
Session Name: Poster Session D: Non-Organ Specific: Pharmacogenomics / Pharmacokinetics
Session Type: Poster Session
Date: Tuesday, June 4, 2019
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall C & D
*Purpose: Evaluate the use of anti-Xa levels in therapeutic enoxaparin dosing in liver/intestine transplant recipients.
*Methods: A single-center retrospective chart review was conducted on liver/intestine transplant recipients hospitalized between July 1, 2016 and July 26, 2018, who received enoxaparin at treatment dose and had anti-Xa levels drawn. Patients were excluded if they received enoxaparin for venous thromboembolism (VTE) prophylaxis or had no anti-Xa levels drawn. Anti-Xa levels were considered therapeutic between 0.5-1.2 U/mL (adults) or 0.5-1.0 U/mL (pediatrics). Levels were considered timed appropriately if collected 3-5 hours after previous dose administration.
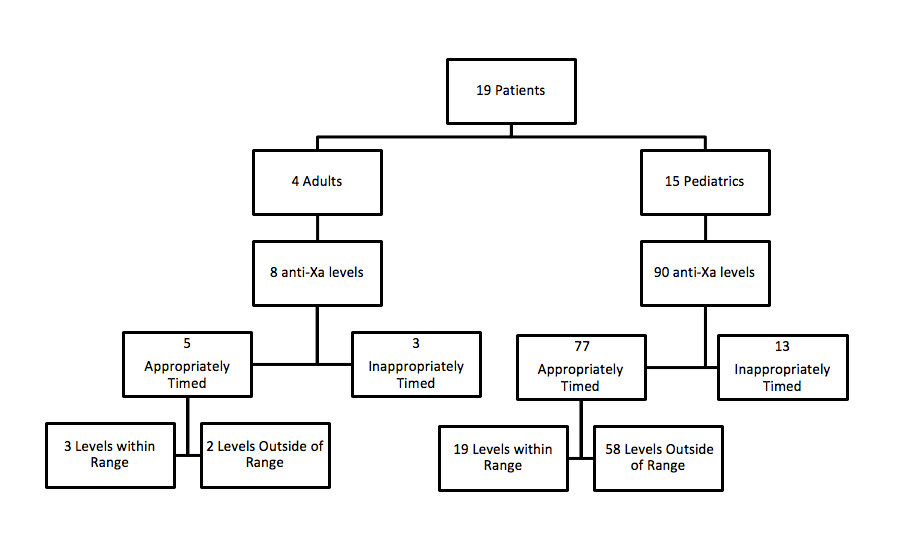
*Results: Four adult and 15 pediatric patients were included. Most were initiated on a dose of 1 mg/kg BID (60% adult; 77% pediatric), which was appropriate per renal function. Ninety-eight anti-Xa levels were drawn: 8 adult, 90 pediatric. Most levels were timed appropriately (63% adult; 86% pediatric), and 27% of levels were within target range. Of the levels outside the target range, all adult levels were supratherapeutic (2/2), and for both, the enoxaparin dose was reduced. Most pediatric levels were subtherapeutic (53/59) and the dose was increased (67%) or remained unchanged (33%). There were no VTE during the study period; however, the rate of bleeding was 37% (7 events: 2 adult, 5 pediatric) with one major bleeding episode. Around the time of bleeding, 3 patients had subtherapeutic levels (2 with recent dose increase), 1 patient had a therapeutic anti-Xa, and 3 patients had no anti-Xa level drawn. Most patients that bled were receiving concomitant aspirin therapy per protocol (71%).
*Conclusions: In our small, retrospective study, 1 mg/kg BID enoxaparin dosing did not result in anti-Xa levels within the target ranges as predicted by pharmacokinetic studies. However, we did not see any VTE despite subtherapeutic levels and the rate of bleeding was higher than expected. The results indicate that standard dosing regimens may not apply to the liver/intestine transplant population and further investigation is necessary to define more appropriate therapy goals.
To cite this abstract in AMA style:
Sullivan J, Jebrock J, Revollo J, Perez M, Garcia J, Beduschi T. Anti-Xa Level Monitoring for Treatment Dose Enoxaparin in Liver/Intestine Transplant Recipients [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/anti-xa-level-monitoring-for-treatment-dose-enoxaparin-in-liver-intestine-transplant-recipients/. Accessed March 6, 2026.« Back to 2019 American Transplant Congress