mTOR Inhibitors Associated with Higher Cardiovascular Adverse Events – A Large Population Database Analysis
1Pharmacy, UC San Diego Health, San Diego, CA, 2Skaggs School of Pharmacy and Pharmaceutical Sciences, San Diego, CA
Meeting: 2019 American Transplant Congress
Abstract number: D208
Keywords: Adverse effects, Arteriosclerosis, FK506, Sirolimus (SLR)
Session Information
Session Name: Poster Session D: Non-Organ Specific: Pharmacogenomics / Pharmacokinetics
Session Type: Poster Session
Date: Tuesday, June 4, 2019
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall C & D
*Purpose: Our study aimed to compare the adverse events (AEs) of tacrolimus to mammalian target of rapamycin (mTOR) inhibitors in solid organ transplant patients.
*Methods: Our study utilized the FDA Adverse Event Reporting System (FAERS) database from 2004 through the 2nd quarter of 2018 to perform a retrospective data analysis. We included patients on immunosuppression for kidney, liver, heart, and lung transplantations. In order to isolate AEs due to specific agents, we analyzed reports due to the individual agents tacrolimus, sirolimus, or everolimus, and compared odds ratios of the mTOR inhibitors to tacrolimus. Duplicate adverse events (often a synonym) in the same report, or reported AEs deemed to be unrelated to medication therapy were excluded. AEs were then grouped into 17 main system-based groups using the MedDRA terminologies.
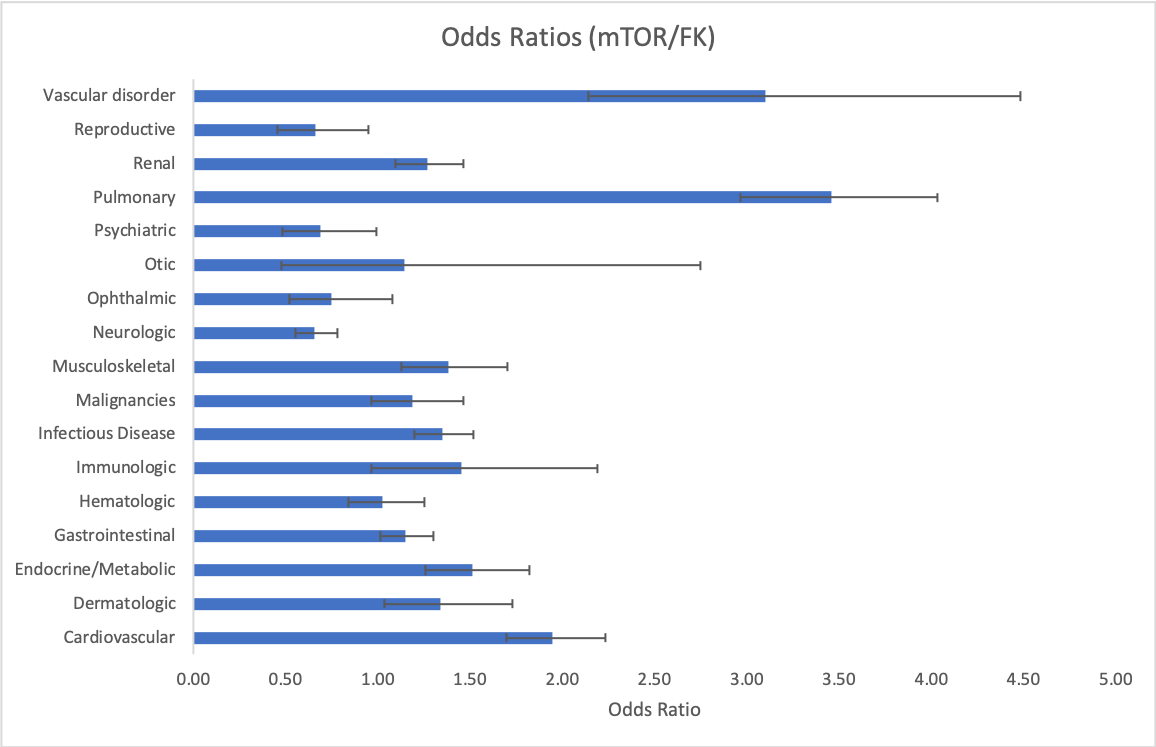
*Results: The mTOR arm had 1282 reports with 4176 AEs while the tacrolimus arm had a total of 7587 reports with 20940 individual AEs. mTOR inhibitors had significantly higher incidences of cardiovascular (OR 1.95, 95% CI 1.70, 2.23), dermatologic (OR 1.34, 95% CI 1.04, 1.73), endocrine (OR 1.52, 95% CI 1.26, 1.82), gastrointestinal (OR 1.15, 95% CI 1.01, 1.30), infectious disease (OR 1.35, 95% 1.20, 1.52), musculoskeletal (OR 1.39, 95% CI 1.13, 1.70), pulmonary (OR 3.46, 95% 2.97, 4.03), renal (OR 1.27, 95% CI 1.10, 1.46), and vascular AEs (OR 3.10, 95% CI 2.14, 4.49). In contrast, mTOR inhibitors had significantly lower reports for neurologic (OR 0.66, 95% CI 0.55, 0.78), psychiatric (OR 0.69, 95% CI 0.48, 0.99), and reproductive (OR 0.66, 95% CI 0.46, 0.95). No differences were found for hematologic, immunologic, malignancies, ophthalmic, and otic. Higher cardiovascular events with mTOR inhibitors was driven mostly by arteriosclerosis, edema, heart failure, and pericardial events. Little is known about the cardiovascular profile of mTOR inhibitors besides edema (everolimus 18-45%, sirolimus 18-20%) and hypertension (everolimus 17-30%, sirolimus 45-49%). The higher incidence rate of arteriosclerosis in the mTOR group has not been reported in the literature and may be explained by dyslipidemia, which is a known complication of mTOR inhibitor therapy.
*Conclusions: We analyzed the adverse event profile of mTOR inhibitors and tacrolimus utilizing a large-scale population database covering 14 years. mTOR inhibitors may be associated with a higher cardiovascular risk. Further analysis is required to determine the potential mechanism of this effect.
To cite this abstract in AMA style:
Nguyen VN, Abagyan R, Tsunoda S. mTOR Inhibitors Associated with Higher Cardiovascular Adverse Events – A Large Population Database Analysis [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/mtor-inhibitors-associated-with-higher-cardiovascular-adverse-events-a-large-population-database-analysis/. Accessed February 19, 2026.« Back to 2019 American Transplant Congress