Natural Course Of Tacrolimus Intra-patient Variability In Liver Transplant Recipients
University of Cincinnati, Cincinnati, OH
Meeting: 2019 American Transplant Congress
Abstract number: D133
Keywords: Calcineurin, Liver
Session Information
Session Name: Poster Session D: Liver: Immunosuppression and Rejection
Session Type: Poster Session
Date: Tuesday, June 4, 2019
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall C & D
*Purpose: The evolution of tacrolimus (TAC) intra-patient variability (IPV) post liver transplant (LT) remains to be characterized. Identification of factors associated with high TAC-IPV may allow for targeted patient screening, early interventions and improved outcomes.
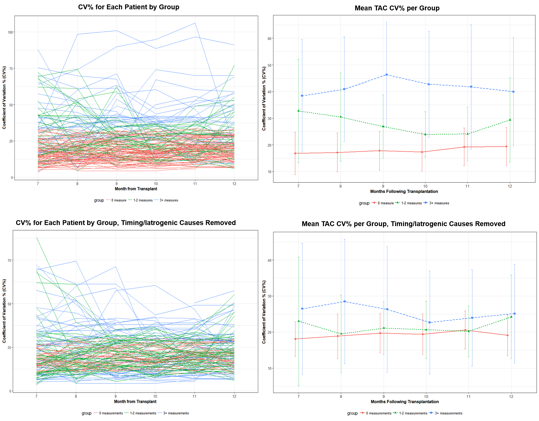
*Methods: LT recipients from 01/2016 to 10/2017 were retrospectively reviewed. Patients with previous transplant or dual organ transplant were excluded. Monthly reports were used to assess TAC-IPV, defined as coefficient of variation (CV%) (standard deviation/mean) using 5 prior ambulatory levels. Elevated CV% was defined as > 35%. Patients were grouped by # of times CV% was > 35% at months 7-12 post-LT: 0 , 1-2 and > 3 measures. Analysis was performed on all time points and on a subset of data that removed time points with elevated CV% due to a cause other than non-adherence (e.g. lab timing error, goal trough change), denoted as “Timing/Iatrogenic Causes Removed.” Natural evolution of monthly TAC IPV was described using means and standard deviation over time. Classification trees and logistic regression were performed to evaluate for factors associated with odds of having 0, 1-2, or 3 or more elevated CV% measures.
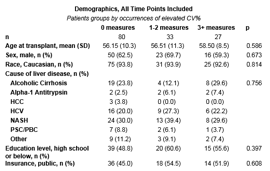
*Results: This analysis included 140 LT patients. Patient demographics are shown in Table 1. The natural course of monthly CV% during months CV% months 7-12 post-LT revealed two subgroups: significant variability of CV%, and stable CV% over time (Figures 1 and 2). With timing/iatrogenic data removed, age at LT, # of medications at 12 months post-LT, and public insurance were strong predictors of elevated CV% at any time point. Odds ratios for age at LT, public insurance, and # of medications were 0.94, 2.13, and 1.1, respectively. No significant predictors were noted when all causes for elevated CV% were included.
*Conclusions: This experience represents the first description of the evolution of TAC-IPV in LT recipients, and a novel approach to TAC-IPV monitoring. Public insurance, age at LT, and # of medications at 12 months post-LT were strong predictors of any elevated CV% 7-12 months post-LT. Importantly, two subgroups of patients with and without high variability in CV% were identified, and may be of particular interest to study.
To cite this abstract in AMA style:
Wilson N, Tremblay S, Ejaz NS, Zheng K, Garrett J, Shah SA, Alloway RR, Kaiser TE. Natural Course Of Tacrolimus Intra-patient Variability In Liver Transplant Recipients [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/natural-course-of-tacrolimus-intra-patient-variability-in-liver-transplant-recipients/. Accessed February 16, 2026.« Back to 2019 American Transplant Congress