Safety Outcomes in Elderly Patients Who Receive Alemtuzumab Induction for Kidney Transplant
Ochsner Clinic Foundation, New Orleans, LA
Meeting: 2019 American Transplant Congress
Abstract number: B199
Keywords: Elderly patients, Induction therapy, Kidney transplantation, Neutropenia
Session Information
Session Name: Poster Session B: Kidney Immunosuppression: Induction Therapy
Session Type: Poster Session
Date: Sunday, June 2, 2019
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall C & D
*Purpose: As a consequence of natural immunosenescence, elderly patients may not require as potent immunosuppression as their younger counterparts. Optimal induction regimens in this population, however, have not been defined. The purpose of this study is to assess the safety and efficacy of alemtuzumab induction in the elderly population.
*Methods: This is a single-center, retrospective, cohort study comparing outcomes in adult kidney transplant recipients who received alemtuzumab for induction at Ochsner Medical Center from January 2013 to December 2017, with a 1-year follow-up. Patients were separated into three groups, based on age at time of transplant: <50 yrs, 50-60 yrs, and >60 yrs. The primary outcome was severe neutropenia, defined as neutropenia requiring G-CSF. Opportunistic infections, incidence of rejection, and patient survival at 1-year were also assessed.
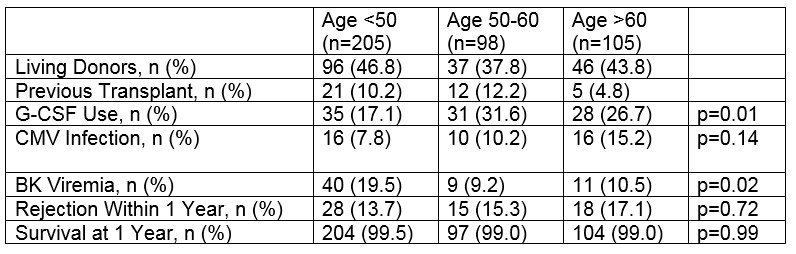
*Results: Recipients in the >60 age group and the 50-60 age group experienced significantly more severe neutropenia, requiring G-CSF, than the patients in the <50 age group. Recipients >60 years of age experienced a higher incidence of CMV infection compared to all other patients (15.2% vs 8.6%%, p=0.05). Interestingly, patients in the youngest cohort had a greater incidence of BK viremia, compared to the 50-60, and >60 age group. There was no difference in rejection or patient survival in the follow-up period. .
*Conclusions: Our experience finds that older patients induced with alemtuzumab suffer from higher rates of CMV and severe neutropenia, without the added benefit of lower rejection rates. Safety risks should be considered when utilizing alemtuzumab for induction in elderly patients.
To cite this abstract in AMA style:
Anders S, Freeman A, Mohammed A, Hutchinson L, Kaszubski U, Janusek M, Garces J, Bohorquez H. Safety Outcomes in Elderly Patients Who Receive Alemtuzumab Induction for Kidney Transplant [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/safety-outcomes-in-elderly-patients-who-receive-alemtuzumab-induction-for-kidney-transplant/. Accessed February 17, 2026.« Back to 2019 American Transplant Congress