Urinary Biomarkers- CXCL9 and CXCL10 for Non-Invasive, Early Diagnosis of Rejection in Pediatric Renal Transplantation
1Pediatrics, Emory University and Children's Healthcare of Atlanta, Atlanta, GA, 2Pediatrics, Emory University, Atlanta, GA
Meeting: 2019 American Transplant Congress
Abstract number: A172
Keywords: Biopsy, Kidney transplantation, Pediatric, Rejection
Session Information
Session Name: Poster Session A: Biomarkers, Immune Monitoring and Outcomes
Session Type: Poster Session
Date: Saturday, June 1, 2019
Session Time: 5:30pm-7:30pm
 Presentation Time: 5:30pm-7:30pm
Presentation Time: 5:30pm-7:30pm
Location: Hall C & D
*Purpose: Renal transplantation is the treatment of choice for End Stage Renal Disease, but requires strict medication adherence and lifelong surveillance for rejection, infection and allograft dysfunction. Renal biopsy remains the gold standard for diagnosing allograft injury but its invasive nature is challenging, especially in children. A compelling need hence exists, for validating non-invasive biomarkers, to detect subclinical rejection early, and allow safe modification of medications based on immunologic risk, to not only prevent rejection, but also reduce excessive medication burden or side effects. Urinary CXCL9 and CXCL10 are two such promising biomarkers, but prospective trials validating their clinical use, especially in children are limited.
*Methods: We performed a cross-sectional analysis of 46 urine samples obtained from 36 pediatric renal transplant recipients, who had kidney biopsies (for-cause or protocol) done at the same time. Urine was analyzed using a solid-phase bead-array assay for the interferon gamma-induced chemokines CXCL9 and CXCL10, in triplicate, using the commercially available CXCL9 and CXCL10 DuoSet ELISA (R&D Systems, Minneapolis, MN) as per manufacturer’s instructions. Urinary chemokine levels were matched to pathologic findings in renal biopsies, classified as acute rejection- borderline or more severe histologic findings (AR), BK nephritis (BKN), or interstitial fibrosis, tubular atrophy (IFTA).
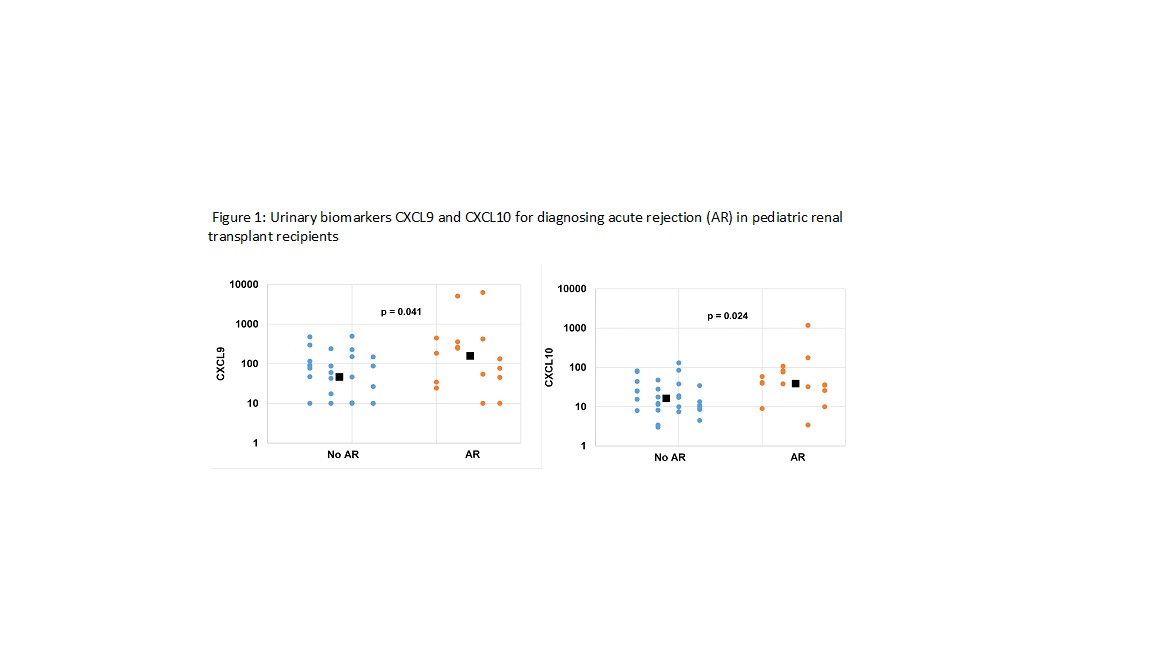
*Results: In children experiencing AR, there was an elevation of both CXCL9 (p=0.041) and CXCL10 (p=0.024) but not in stable allograft recipients, or recipients with IFTA (Figure 1). Only 1 patient had biopsy proven BKN and showed increase in both biomarkers, as compared to patients with stable allograft. Using cut-point analysis for AR, BK viremia and donor specific antibody, we determined values for CXCL9 and CXCl10 to identify patients with allograft inflammation, with 81% sensitivity and 84% negative predictive value.
*Conclusions: These data show that urine chemokine monitoring can help non-invasively screen and identify pediatric renal transplant recipients with renal allograft inflammation. In the future, we plan to use CXCL9 and CXCl10 cut-points to stratify patients into immune-quiescent or immune-active groups, to predict outcomes and either lower medication burden or escalate monitoring/therapy.
To cite this abstract in AMA style:
George RP, Hanberry B, Dave I, Lee B, Garro R, McCracken C, Warshaw B. Urinary Biomarkers- CXCL9 and CXCL10 for Non-Invasive, Early Diagnosis of Rejection in Pediatric Renal Transplantation [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/urinary-biomarkers-cxcl9-and-cxcl10-for-non-invasive-early-diagnosis-of-rejection-in-pediatric-renal-transplantation/. Accessed January 28, 2026.« Back to 2019 American Transplant Congress