Parathyroid CD34+ Cells Induce Neovascularization of Donor and Recipient Leading to Chimeric Vessel Formation and Improved Engraftment of Co-Transplanted Pancreatic Islets
1UCSF, San Francisco, CA, 2San Francisco Veterans Affairs, San Francisco, CA
Meeting: 2019 American Transplant Congress
Abstract number: A126
Keywords: Engraftment, Ischemia
Session Information
Session Name: Poster Session A: Islet Cell and Cell Transplantation
Session Type: Poster Session
Date: Saturday, June 1, 2019
Session Time: 5:30pm-7:30pm
 Presentation Time: 5:30pm-7:30pm
Presentation Time: 5:30pm-7:30pm
Location: Hall C & D
*Purpose: We recently showed improved engraftment and diabetes reversal when mature islets or stem-cell-derived beta cells (SCIPC) were co-transplanted with parathyroid gland (PTG). The CD34+ vascular endothelial progenitor cells, comprising 3-5% of PTG, recapitulated this effect whereas the CD34- cells were less effective. This study aims to test the hypothesis that PTG, particularly the CD34+ cells, protect islets by accelerating and enhancing neovascularization.
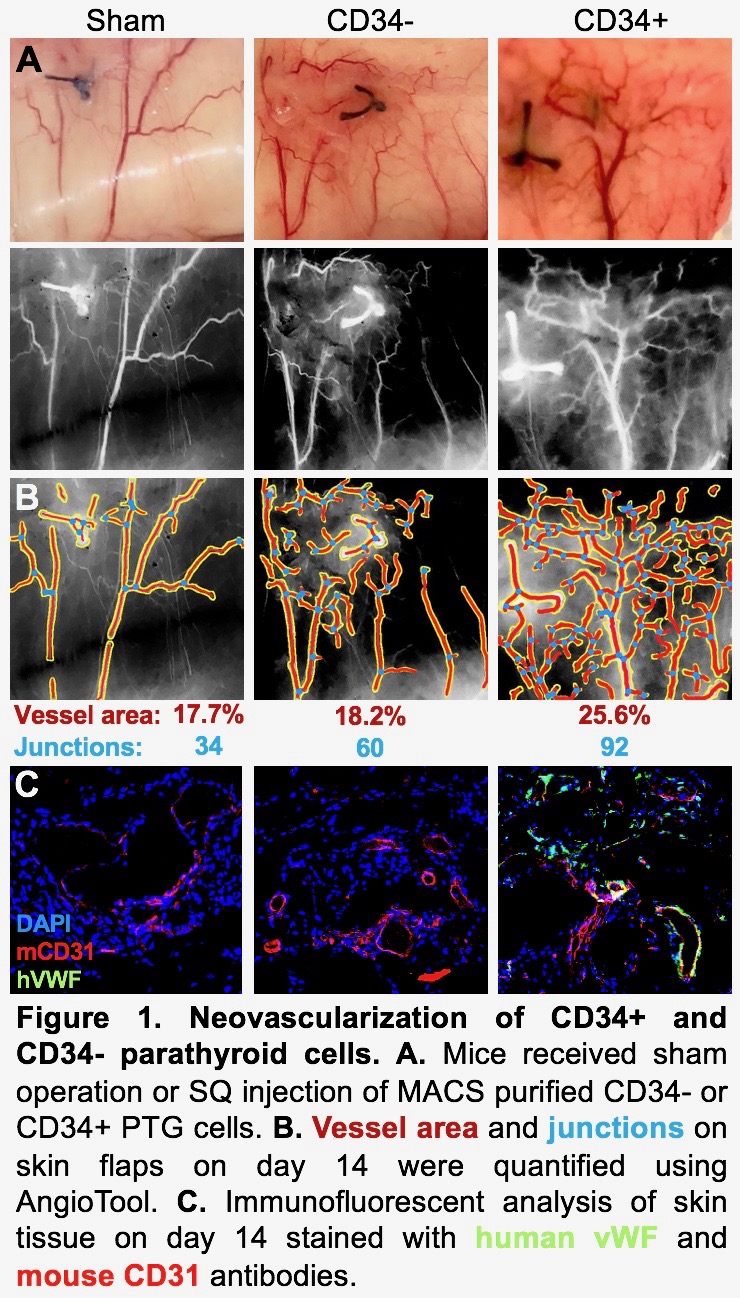
*Methods: Human PTG was dissociated into single cells using enzymatic digestion. CD34+ and CD34- cells were purified using magnetic-activated cell sorting. One fourth of a PTG, 1,000 IEQ human islets or 150,000 PTG CD34+ or CD34- cells were transplanted in the subcutaneous space (SQ) of immunodeficient NSG mice. Sham controls received incisions without transplants. Skin flaps surrounding the transplant sites were created to reveal the vasculature at day 5 and 14 post-transplant. Vessel area percentage and number of vascular junctions were quantified using AngioTool (NIH). Skin tissue was prepared for histology by fixing in 4% PFA and embedding in OCT. Cryosections were stained with anti-human von Willebrand factor (vWF) antibody conjugated to Alexa 488, anti-mouse CD31 conjugated to Alexa 647 and DAPI and imaged with confocal microscopy.
*Results: At 5 days post-transplant, skin flaps with PTG CD34+ transplanted cells had 29.1% vessel area with 103 junctions. In comparison, PTG CD34- flaps had 22.9% vessel area and 62 junctions and sham flaps had 19.5% vessel area and 54 junctions. At 14 days post-transplant, CD34+ flaps had 25.6% vessel area and 92 junctions, CD34- flaps had 18.2% vessel area and 60 junctions, and sham flaps had 17.7% vessel area and 34 junctions. Histologic assessment of human PTG and CD34+ cell transplants at 14 days showed an abundance of human vWF staining, demonstrating human-derived vessels. These human vessels were surrounded by mouse vessels identified by mouse CD31 staining. Human vWF and mouse CD31 signals were observed in same vessels, suggesting human/mouse chimeric vessel formation. In contrast, the sham had no human blood vessels and the islet and CD34- transplants were dominated by mouse vessels (Figure 1).
*Conclusions: PTG, and in particular the CD34+ cells, induced early and sustained neovascularization as evidenced by increased vessel area percentage and vascular junctions, compared with sham, islet and CD34- transplants. While the vascular network of PTG CD34- and islet transplants is almost exclusively recipient derived, that of PTG and PTG CD34+ transplants is comprised of both recipient and donor tissue. This rich chimeric network may protect islets from ischemic injury and may be the mechanism by which co-transplantation of PTG or its CD34+ component improves engraftment.
To cite this abstract in AMA style:
Kelly Y, Ward C, Duh Q, Chang W, Stock P, Tang Q. Parathyroid CD34+ Cells Induce Neovascularization of Donor and Recipient Leading to Chimeric Vessel Formation and Improved Engraftment of Co-Transplanted Pancreatic Islets [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/parathyroid-cd34-cells-induce-neovascularization-of-donor-and-recipient-leading-to-chimeric-vessel-formation-and-improved-engraftment-of-co-transplanted-pancreatic-islets/. Accessed February 28, 2026.« Back to 2019 American Transplant Congress