Hepatitis C Treatment and Transplant Outcomes in Liver Transplant Recipients with Hepatitis C Positive Donors
I. Booth, J. Clark, J. C. LaMattina, A. Haririan, R. N. Barth, B. Ravichandran
University of Maryland Medical Center, Baltimore, MD
Meeting: 2019 American Transplant Congress
Abstract number: 587
Keywords: Graft survival, Hepatitis C, Liver
Session Information
Session Name: Concurrent Session: Non-Organ Specific: Viral Hepatitis
Session Type: Concurrent Session
Date: Tuesday, June 4, 2019
Session Time: 4:30pm-6:00pm
 Presentation Time: 4:54pm-5:06pm
Presentation Time: 4:54pm-5:06pm
Location: Room 309
*Purpose: With direct-acting antivirals for hepatitis C virus (HCV), transplant centers have expanded the donor pool with positive short term outcomes with early HCV treatment. However, the impact of HCV donor status on NAT positive liver transplant recipient (LTR) outcomes has not been well described.
*Methods: This retrospective chart review included HCV NAT positive adults who received deceased donor liver transplants alone between 2013-2017 stratified into 3 cohorts by donor: NAT positive, Ab positive and NAT negative and Ab and NAT negative. All LTRs received standard immunosuppression with tacrolimus, mycophenolate and a 3-day steroid taper. The primary endpoint was one year graft survival. Secondary endpoints included rejection episodes at any time point and HCV treatment outcomes, including SVR and time to treatment post-transplant.
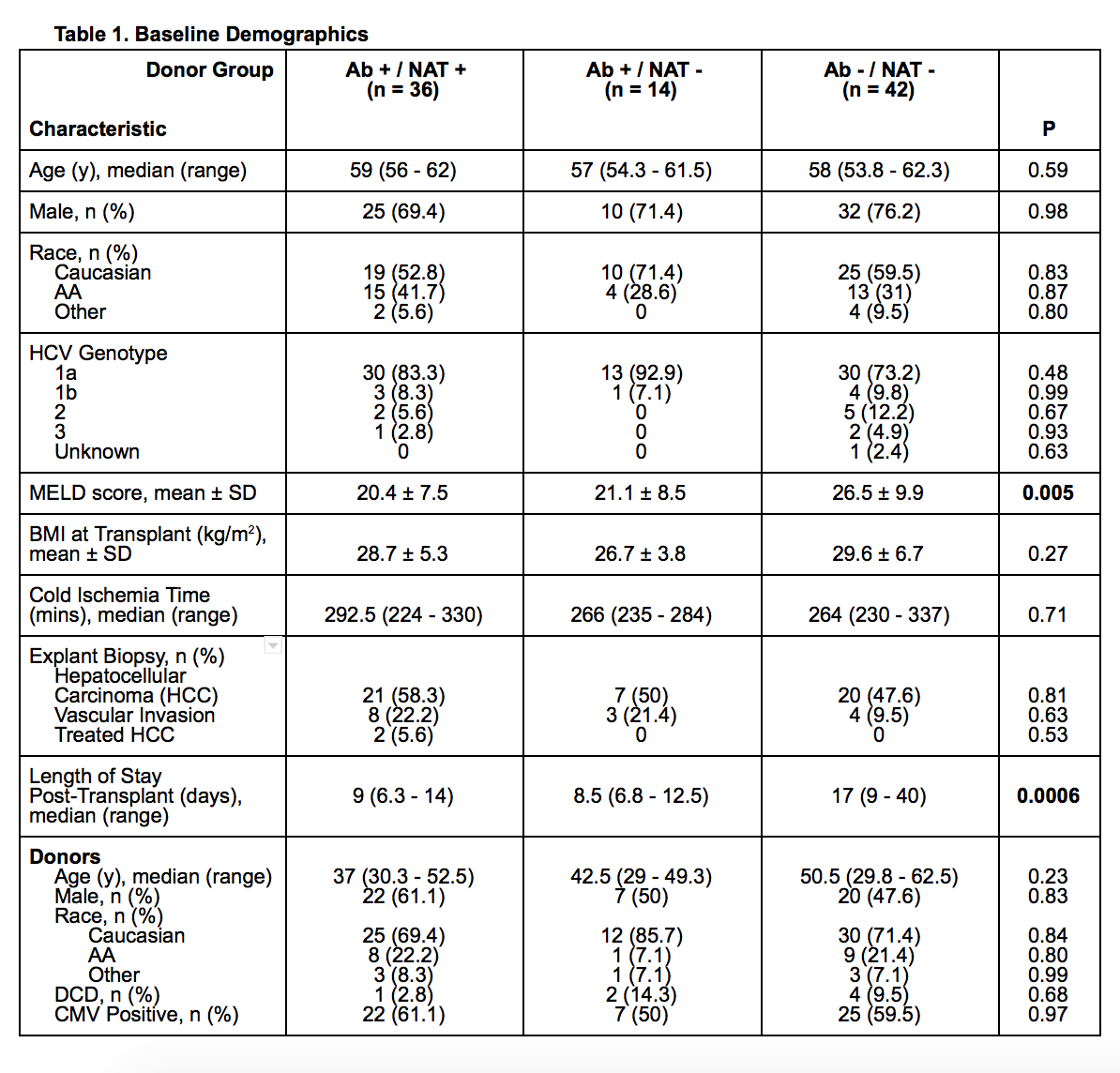
*Results: Of 92 LTRs, 36 had NAT positive donors, 14 had Ab positive and NAT negative donors and 42 had Ab and NAT negative donors. Baseline demographics are reported in Table 1. Of note, median length of stay and MELD score was significantly lower in the HCV positive groups compared to the negative group. There was no difference in one year graft survival or median time to graft loss (Table 2). Of patients treated for HCV, time to treatment was 171 (126 – 223) days, 175 (128.5 – 222.3) days, and 197.5 (105.5 – 272.5) days, respectively (p=0.82), with no failures. There were no clinical differences between tacrolimus trough concentrations or mycophenolate dose at any time point. Median time to last follow up was 699.3, 727.3, and 763.7 days, respectively.
*Conclusions: While HCV donor status does not impact graft survival at one year in HCV positive LTRs, HCV positive donors may accommodate transplant at a lower MELD score. Delayed time to HCV treatment did not impact outcomes in this cohort.
To cite this abstract in AMA style:
Booth I, Clark J, LaMattina JC, Haririan A, Barth RN, Ravichandran B. Hepatitis C Treatment and Transplant Outcomes in Liver Transplant Recipients with Hepatitis C Positive Donors [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/hepatitis-c-treatment-and-transplant-outcomes-in-liver-transplant-recipients-with-hepatitis-c-positive-donors/. Accessed February 26, 2026.« Back to 2019 American Transplant Congress