Intravenous Immunoglobulin Significantly Reduces Bioavailability of Concomitantly Administered Anti-C5 Monoclonal Antibodies
1Comprehensive Transplant Center, Cedars-Sinai Medical Ctr, Los Angeles, CA, 2Novartis Institutes for BioMedical Research, Basel, Switzerland, 3Biometrics Matters Ltd, Hamilton, New Zealand, 4Novartis Institutes for BioMedical Research, Cambridge, MA
Meeting: 2019 American Transplant Congress
Abstract number: 399
Keywords: Drug interaction, IVIG, Monoclonal antibodies, Pharmacokinetics
Session Information
Session Name: Concurrent Session: Non-Organ Specific: Pharmacogenomics / Pharmacokinetics
Session Type: Concurrent Session
Date: Monday, June 3, 2019
Session Time: 4:30pm-6:00pm
 Presentation Time: 5:42pm-5:54pm
Presentation Time: 5:42pm-5:54pm
Location: Room 210
*Purpose: Awareness of drug-drug interactions is critical in management of organ transplant recipients. However, there is little understanding of the possibility of biologic agents interfering with the efficacy of other concomitantly administered monoclonal antibodies. Here, the use of high dose intravenous immune globulin (IVIg) for immune modulation may have an important impact.
*Methods: This was a single center open label, parallel group, partially randomized, non-confirmatory study to explore a potential effect of IVIg on the PK and PD of the human anti-C5 monoclonal (LFG316). The study investigated the safety, tolerability, pharmacokinetics and pharmacodynamics of LFG316+IVIg group compared to LFG316 alone.
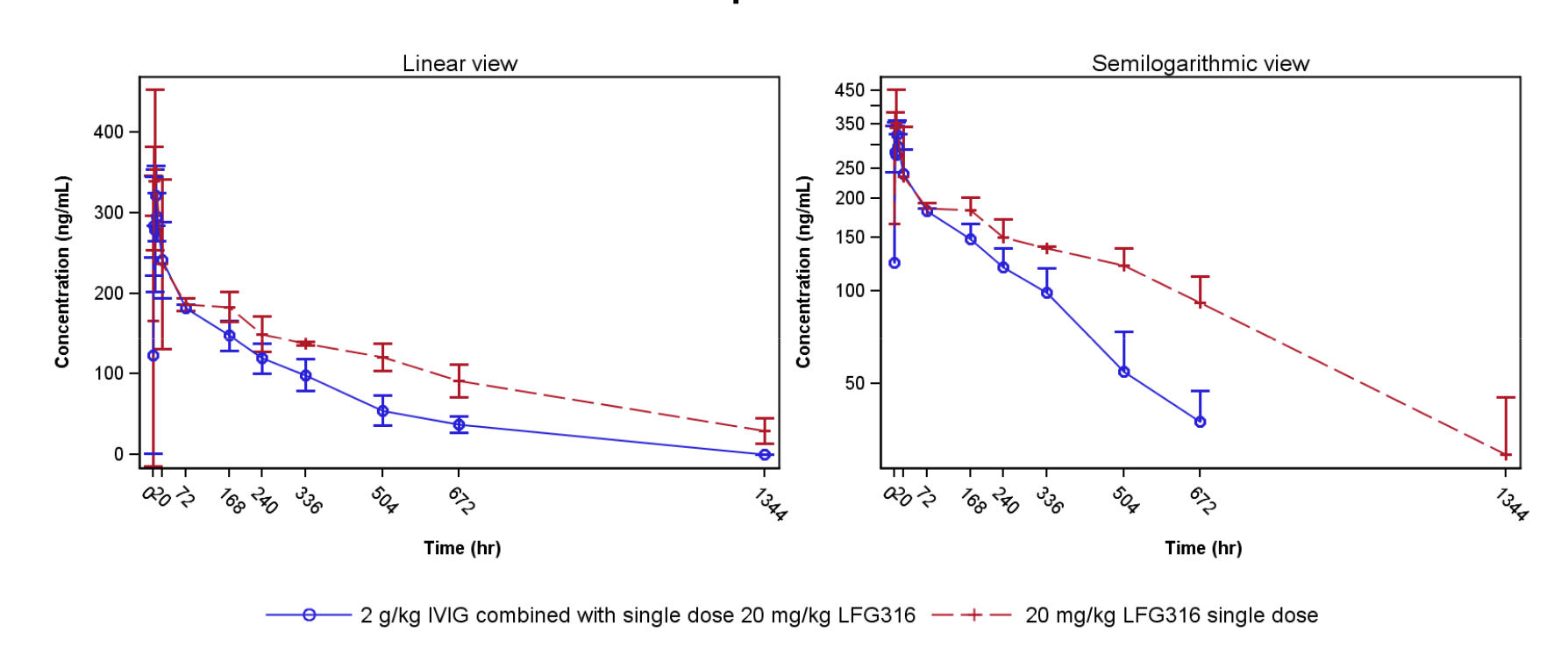
*Results: The pharmacokinetics of LFG316 was evaluated separately for the two groups where LFG316 was administered immediately after high dose IVIg (n=6) and LFG316 alone (n=2). Despite the small sample size, the PK parameters obtained from both groups were consistent and with low variability. In the LFG316+IVIg group, the mean value for AUCinf/D decreased by 34%, clearance increased by 63% and t1/2 decreased by 41% compared with the LFG316 alone group. This difference in clearance/elimination characteristics between the treatment arms was evident from 3 days to 21 days where faster elimination of LFG316 was observed. This data is shown in figure 1 below. In the subsequent weeks of the observation period, the influence on clearance by the IVIg infusion diminished. LFG316 suppressed the complement pathway as measured by the Wieslab assay and CH50 in serum. The rapid elimination of LFG316 in the LFG316+IVIg group allowed more rapid recovery of complement activity.
*Conclusions: The infusion treatments LFG316 alone and LFG316+IVIg were found to be safe and well tolerated. High dose IVIg infusion administered immediately before infusion of LFG316 has a significant impact on the pharmacokinetics and pharmacodynamics of LFG316 resulting in a shortening of the period in which full inhibition of complement activity is maintained. The effect of high dose IVIg on LFG316 clearance is most profound during the first 2 weeks after IVIg infusion. These findings suggest a significant impact of IVIg on clearance of monoclonal antibodies that limits efficacy. This is likely through occupation of the Fc neonatal receptor (FcRn) by high dose IVIg, increasing clearance of monoclonals. These findings also reveal an important therapeutic pathway for IVIg administration in enhancing elimination of pathogenic antibodies in humans.
To cite this abstract in AMA style:
Jordan SC, Kucher K, Bagger M, Hockey H, Wagner K, Ammerman N, Choi J, Vo A. Intravenous Immunoglobulin Significantly Reduces Bioavailability of Concomitantly Administered Anti-C5 Monoclonal Antibodies [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/intravenous-immunoglobulin-significantly-reduces-bioavailability-of-concomitantly-administered-anti-c5-monoclonal-antibodies/. Accessed February 23, 2026.« Back to 2019 American Transplant Congress