Impact of Liver Recipient Genotype on Tacrolimus
1University of Cincinnati, Cincinnati, OH, 2Cincinnati Children's Hospital and Medical Center, Cincinnati, OH, 3iC42 Clinical Research and Development, Aurora, CO
Meeting: 2019 American Transplant Congress
Abstract number: 394
Keywords: Genomics, Immunosuppression, Liver, Pharmacokinetics
Session Information
Session Name: Concurrent Session: Non-Organ Specific: Pharmacogenomics / Pharmacokinetics
Session Type: Concurrent Session
Date: Monday, June 3, 2019
Session Time: 4:30pm-6:00pm
 Presentation Time: 4:42pm-4:54pm
Presentation Time: 4:42pm-4:54pm
Location: Room 210
*Purpose: In kidney transplant recipients, recipient genotype (CYP3A5 *3) has the greatest influence on tacrolimus pharmacokinetics (PK). However, in liver transplant recipient, donor liver and recipient gut genotypes may differ. The purpose of this study was to evaluate the impact of donor and recipient genotype in adult, deceased donor North American stable liver transplant recipients more than 6 months from transplant.
*Methods: 12-hour PK profiles (2/patient) were obtained from stable liver transplants (U01 FD004573, PI. Alloway). Recipients were genotyped for CYP3A5 *3 (rs776746), ABCB1 3435C>T (rs1045642) and POR*28 (rs1057868). CL/F, AUC/dose, dose, Ctrough and Ctrough/dose were previously derived using Winnonlin. Kruskall-Wallis tests were performed between genotypes, random forests trees and recursive feature selection were used to select variables in the final mixed effects model and known factors, including age, sex, race, height, weight, BMI, hematocrit, MMF, steroids, diabetes were included. Final mixed effects model selection was done using the Akaike Information Criterion.
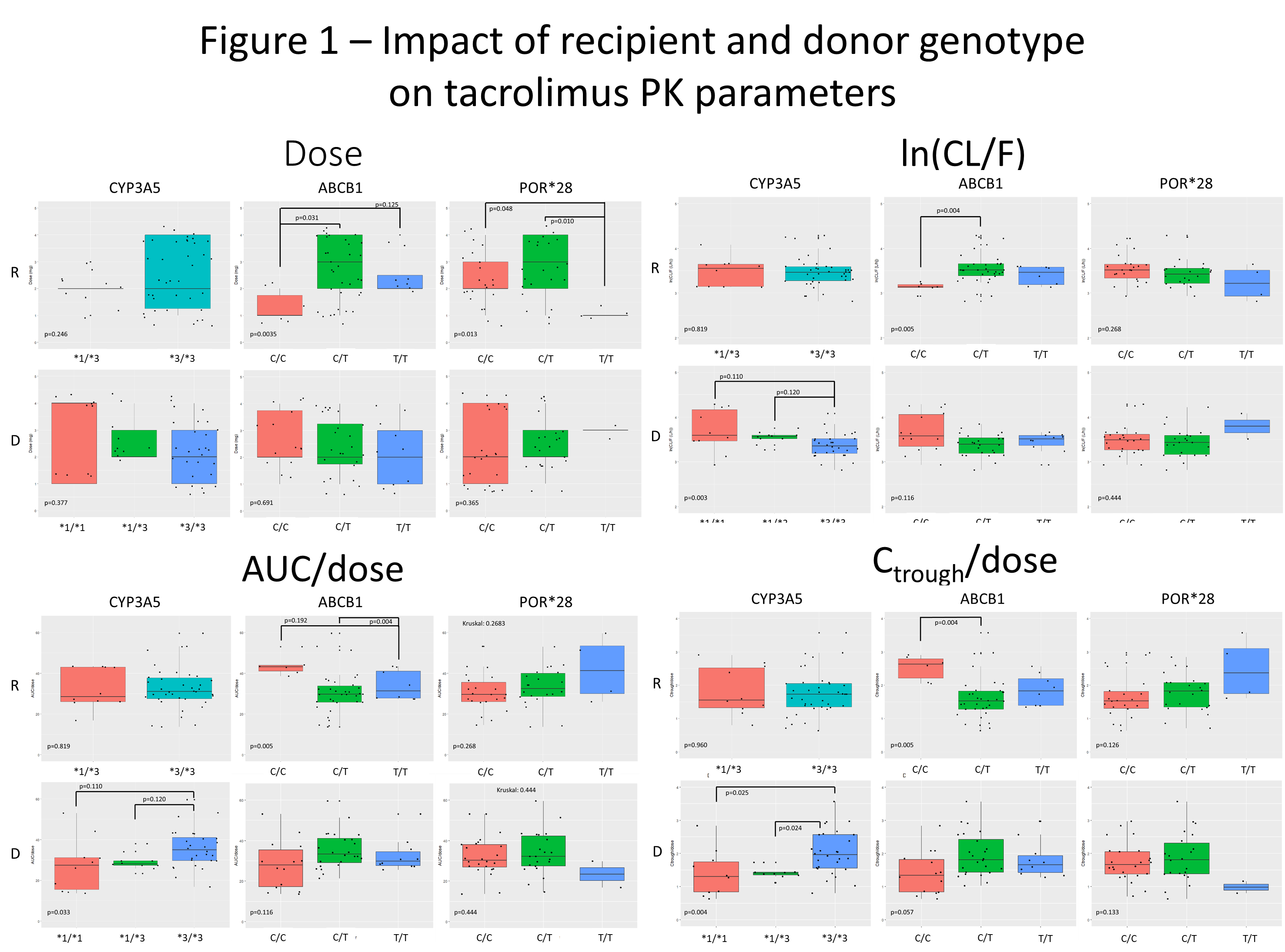
*Results: 24 donors had available genetic material. Recipient ABCB1 T resulted in significantly higher CL/F and dose, lower AUC/dose and Ctrough/dose. Donor CYP3A5*3 was associated with lower CL/F, higher AUC/dose and Ctrough/dose but not with dose (Figure 1).
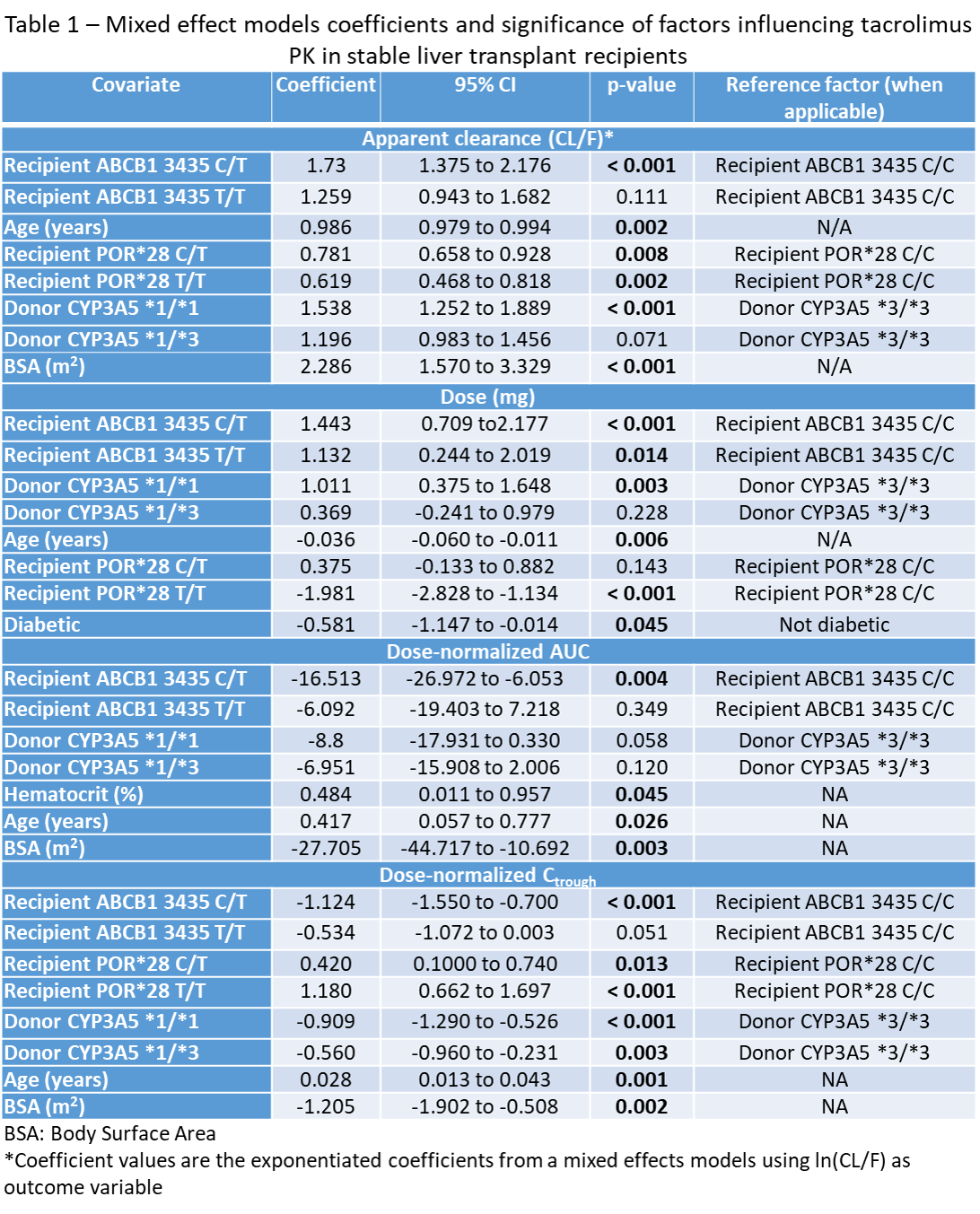
Final mixed effects models revealed that recipient ABCB1, age, donor CYP3A5, diabetes were associated with CL/F, dose, AUC/dose, and Ctrough/dose. Age, diabetes, hematocrit, and BSA (post-hoc) were associated with some of the parameters (Table 1).
Race, MMF, steroids were not associated with changes in PK parameters. Models were able to explain up to 75% of the variance in the data.
*Conclusions: In stable liver transplant recipients more than 6 months from transplant, recipient ABCB1 (gut) and donor CYP3A5 (liver) were significantly associated with changes in PK parameters, whereas race was not. Interactions between recipient (gut) and donor (liver) genotypes explain significant variability in stable liver transplant recipients.
To cite this abstract in AMA style:
Tremblay S, Fukuda T, Mizuno T, Vinks AA, Woodle E, Klawitter J, Klawitter J, Christians U, Alloway RR. Impact of Liver Recipient Genotype on Tacrolimus [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/impact-of-liver-recipient-genotype-on-tacrolimus/. Accessed March 2, 2026.« Back to 2019 American Transplant Congress