Not All Donor-Specific Antibodies Are Equal: Predictors of Risk of Chronic Lung Allograft Dysfunction
1Pharmacy and Therapeutics, University of Pittsburgh, Pittsburgh, PA, 2Florida Hospital Transplant Institute, AdventHealth Orlando, Orlando, FL, 3Pathology, University of Pittsburgh, Pittsburgh, PA, 4Medicine, University of Pittsburgh, Pittsburgh, PA
Meeting: 2019 American Transplant Congress
Abstract number: 390
Keywords: Alloantibodies, Graft failure, Lung transplantation
Session Information
Session Name: Concurrent Session: Infections, Antibodies and Biomarkers in Lung Transplantation
Session Type: Concurrent Session
Date: Monday, June 3, 2019
Session Time: 4:30pm-6:00pm
 Presentation Time: 5:18pm-5:30pm
Presentation Time: 5:18pm-5:30pm
Location: Room 206
*Purpose: Donor-specific HLA antibodies (DSA) have been previously associated with chronic lung allograft dysfunction (CLAD). However, the DSA characteristics that predict risk of CLAD are not well defined. The purpose of this investigation was to identify DSA characteristics associated with increased risk for CLAD in lung transplant recipients (LTRs).
*Methods: In this single-center, retrospective cohort study, adult LTRs were tested by Luminex Single Antigen Bead (SAB) IgG and complement-binding (C1q) assays. IgG-SAB MFI > 1000 and C1q-SAB MFI > 500 were considered positive. Outcomes included CLAD, as well as CLAD subtypes of bronchiolitis obliterans syndrome (BOS) and restrictive allograft syndrome (RAS). Outcomes were assessed using Kaplan-Meier methods, logistic regression, and multivariable Cox regression with a time-dependent covariate.
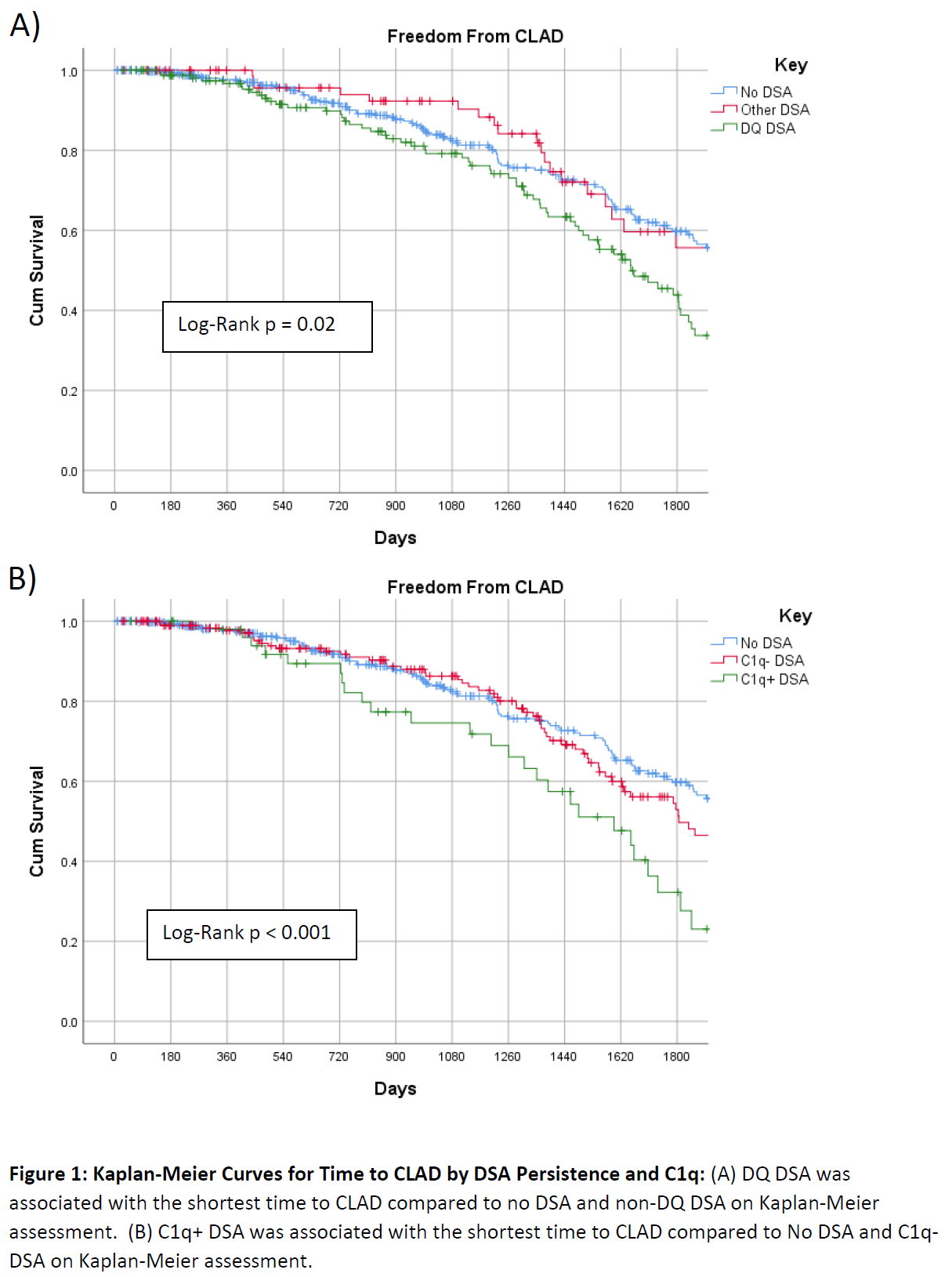
*Results: 582 LTRs were analyzed. 335 (57.6%) had no DSA, 194 (33.3%) C1q- DSA, and 53 (9.1%) C1q+ DSA. Of 247 LTR with DSA, Class II DSA (206 patients, 84%) were more common than Class I DSA (106, 43%). DQ antibody was most common (114, 59% of DSA+ LTRs) and more likely to be C1q+ than non-DQ antibody (OR: 7.01 95%CI: 3.88-12.66). Having both Class I and Class II DSAs was associated with increased hazard of CLAD compared to no DSA on multivariable assessment (HR: 2.16 95%CI: 1.39-3.34). No significant differences were seen with Class I or Class II alone. Consistent with the Kaplan-Meier analysis in Figure 1A, DQ DSA was associated with increased hazard of CLAD on multivariable analysis when compared to no DSA (HR: 1.64 95%CI: 1.20-2.23) and non-DQ DSA (HR:1.66 95%CI: 1.05-2.65). C1q+ DSA had shorter time to CLAD vs no DSA and C1q- DSA on multivariable analysis (HR:2.10 95%CI: 1.34-3.31) (Figure 1B). Similar findings were observed for BOS (HR:2.02 95% CI: 1.26-3.25) and RAS (HR: 2.15 95% CI: 1.30-3.57).
*Conclusions: DSA characteristics defining high risk of CLAD include complement binding and DQ specificity.
To cite this abstract in AMA style:
Iasella CJ, Ensor CR, Marrari M, Mangiola M, Moore CA, Morrell MR, Pilewski J, McDyer JF, Zeevi A. Not All Donor-Specific Antibodies Are Equal: Predictors of Risk of Chronic Lung Allograft Dysfunction [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/not-all-donor-specific-antibodies-are-equal-predictors-of-risk-of-chronic-lung-allograft-dysfunction/. Accessed March 9, 2026.« Back to 2019 American Transplant Congress