Mitochondrial Dysfunction and Metabolic Reprogramming: Critical Features of Cyclosporine A Nephrotoxicity
University of Alabama at Birmingham, Birmingham, AL
Meeting: 2019 American Transplant Congress
Abstract number: 261
Keywords: Calcineurin, Immunosuppression, Kidney, Toxocity
Session Information
Session Name: Concurrent Session: Immunosuppression Preclinical Studies
Session Type: Concurrent Session
Date: Monday, June 3, 2019
Session Time: 2:30pm-4:00pm
 Presentation Time: 2:42pm-2:54pm
Presentation Time: 2:42pm-2:54pm
Location: Room 309
*Purpose: Calcineurin inhibitors (CNIs) are potent immunosuppressants but over time contribute to allograft fibrosis. Clinical efforts to minimize or avoid CNI have not been uniformly successful. Mitigating the nephrotoxicity of CNI is a clinically relevant strategy to facilitate their chronic use. Herein, we investigated a novel link between CNI nephrotoxicity and impaired mitochondrial bioenergetic homeostasis and metabolic reprogramming as a potential strategy to ameliorate CNI renal injury.
*Methods: In addition to proximal tubular epithelial cells (PTECs) for in vitro studies, we utilized a mouse model of CsA acute nephrotoxicity. Mice received a 0.1% NaCl diet for 7 days, followed by CsA 90 mg/kg IP daily for 1 week. Mitochondrial function and structure were determined using Seahorse™ analyzer and western blot analysis of major components in mitochondrial electron chain complexes. Mouse kidney homogenates and PTECs were also analyzed for expression of mTOR, β-catenin, AMPK, ERK1/2 and PI3K/Akt signaling.
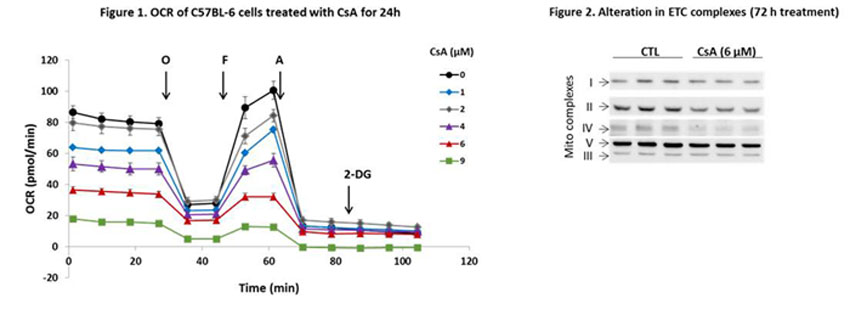
*Results: Exposure of PTECs to CsA resulted in dose dependent decrease in mitochondrial oxygen consumption rates (OCR). Notably, significant decreases of OCR basal, maximal, ATP-linked, and mitochondrial reserve capacity were elicited by non-apoptotic concentrations of CsA (Figure 1). The CsA-mediated effects were associated with significant decrease of NDUFB8 (complex I), FeS comp I (complex II), and subunit I of complex IV (Figure 2). Such loss of mitochondrial indices was accompanied by bioenergetic reprogramming of PTECs. While oxidative phosphorylation was diminished, we observed an increase of extracellular acidification rates (ECAR), indicative of enhanced glycolysis. Metabolic reprogramming and remodeling was accompanied by significant activation of mTOR, along with activation of anabolic and pro-remodeling signaling related to MAPK ERK1/2 and PI3K/Akt phosphorylation and accumulation of β-catenin. Importantly, similar alterations, including mitochondrial, bioenergetic remodeling and epithelial injury, were observed in a mice model of CsA-mediated nephrotoxicity.
*Conclusions: Our results indicate that acute exposure of PTECs to CsA results in a loss of oxidative phosphorylation, with bioenergetic reprogramming to glycolytic metabolism as an initial adaptive (pro-survival) response. However, these responses culminate in metabolic and bioenergetic maladaptation and renal epithelial cell dysfunction. Disruption and return to cellular homeostasis may be one strategy to ameliorate CsA nephrotoxicity.
To cite this abstract in AMA style:
Zmijewska AA, Benavides G, Darley-Usmar V, Zmijewski JW, Mannon RB. Mitochondrial Dysfunction and Metabolic Reprogramming: Critical Features of Cyclosporine A Nephrotoxicity [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/mitochondrial-dysfunction-and-metabolic-reprogramming-critical-features-of-cyclosporine-a-nephrotoxicity/. Accessed February 12, 2026.« Back to 2019 American Transplant Congress