Mismatched Epitope and De Novo DSA Development: An Analysis of the Benefit and Benefit-EXT Renal Transplant Cohorts
1Terasaki Research Institute, Los Angeles, CA, 2Emory University, Atlanta, GA, 3Bristol-Myers Squibb, Princeton, NJ
Meeting: 2019 American Transplant Congress
Abstract number: 159
Keywords: Epitopes, HLA antibodies, Immunogenicity, Kidney transplantation
Session Information
Session Name: Concurrent Session: Histocompatibility and Immunogenetics
Session Type: Concurrent Session
Date: Sunday, June 2, 2019
Session Time: 4:30pm-6:00pm
 Presentation Time: 4:30pm-4:42pm
Presentation Time: 4:30pm-4:42pm
Location: Room 309
*Purpose: De novo donor-specific HLA antibodies (dnDSA) target epitopes rather than whole antigens, and their development is associated with the mismatched HLA antibody epitope load (EpiL) of a donor-recipient pair. We evaluated, across different immunosuppression treatments, the impact of the EpiL on the development of dnDSA.
*Methods: We studied 217 kidney transplant recipients with at least one donor-recipient serological HLA mismatch (MM) for HLA-A/B/DRB1/DQB1, who received cyclosporine (n=71) or belatacept (n=146) as first-line immunosuppression in the BENEFIT and BENEFIT-EXT trials. The absolute proportions of HLA MM that induced the development of dnDSA by year 7 post-transplantation were assessed using a univariate Cox proportional hazard model. Hazard ratios (HR) were described by their point estimate and corresponding 95% confidence interval (CI). In addition, we used E3 – an epitope analysis software developed by the Terasaki Research Institute – to convert/impute serological typing to high-resolution typing and to estimate the EpiL of each donor-recipient pair.
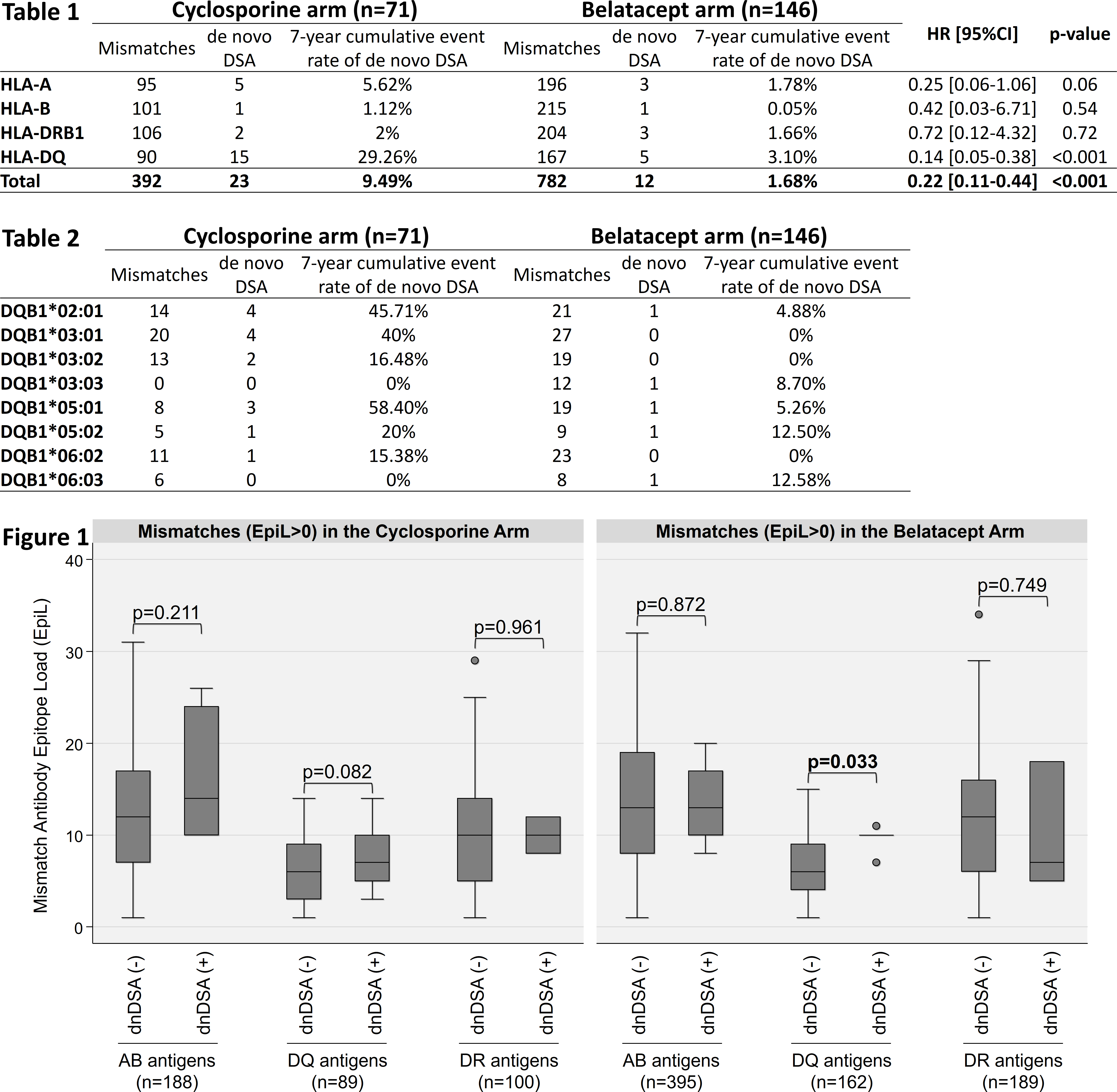
*Results: We confirmed that the 7-year cumulative event rate of dnDSA was significantly lower for MM patients treated with belatacept compared to cyclosporine (p<0.001, HR [95%CI] = 0.22 [0.11-0.44]), where the 7-year cumulative event rate of DQB1 dnDSA was significantly impacted (p<0.001, HR [95%CI] = 0.14 [0.05-0.38]), but not that for A, B, or DRB1 dnDSA (Table1). The DQB1 MM with highest 7-year cumulative event rates were: DQB1*05:01 (58%), DQB1*02:01 (46%), and DQB1*03:01 (40%) in the cyclosporine arm; and DQB1*06:03 (13%), DQB1*05:02 (13%), and DQB1*03:03 (9%) in the belatacept arm (Table 2). Among the MM in the cyclosporine arm, there was no statistical difference in the EpiL between MM that induced a dnDSA and MM that did not for A/B, DRB1, and DQB1 epitopes. Among the MM in the belatacept arm, DQ MM that induced a dnDSA had a significantly higher EpiL compared to DQ MM that did not induce a dnDSA (p=0.033), while there was no statistical difference in the EpiL between MM that did or did not induce a dnDSA for A/B, or DRB1 epitopes (Figure 1).
*Conclusions: Compared to cyclosporine, the use of belatacept in DQB1 MM patients may reduce the development of DQB1 dnDSA. The DQB1 EpiL is associated with development of DQB1 dnDSA and may help predict development of dnDSA under belatacept, but not cyclosporine therapy.
To cite this abstract in AMA style:
Jucaud V, Burnett V, Gebel H, Bray R, Polinsky M, Everly M. Mismatched Epitope and De Novo DSA Development: An Analysis of the Benefit and Benefit-EXT Renal Transplant Cohorts [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/mismatched-epitope-and-de-novo-dsa-development-an-analysis-of-the-benefit-and-benefit-ext-renal-transplant-cohorts/. Accessed February 22, 2026.« Back to 2019 American Transplant Congress