Ex-Vivo Perfusion Mediated Delivery of Rituximab to Clear Latent Epstein-Barr Virus
T. Ku, M. Cypel, R. V. Ribeiro, V. H. Ferreira, D. Kumar, A. Humar
UHN, Toronto, ON, Canada
Meeting: 2019 American Transplant Congress
Abstract number: 53
Keywords: Epstein-Barr virus (EBV), Lung transplantation, Post-transplant lymphoproliferative disorder (PTLD)
Session Information
Session Name: Concurrent Session: PTLD/Malignancies: All Topics
Session Type: Concurrent Session
Date: Sunday, June 2, 2019
Session Time: 2:30pm-4:00pm
 Presentation Time: 3:42pm-3:54pm
Presentation Time: 3:42pm-3:54pm
Location: Room 311
*Purpose: Post-transplant lymphoproliferative disorder (PTLD) can manifest as a B-cell malignancy associated with Epstein-Barr virus (EBV) infection. Risk of PTLD is greatest in EBV D+/R- recipients where latent virus can reactivate within the allograft. Ex-vivo lung perfusion (EVLP) is a promising platform to precondition grafts for transplant. We hypothesized that EVLP mediated delivery of Rituximab (RTX), an antibody targeting CD20+ B cells, could reduce the burden of latent EBV residing in donor lungs thereby reducing risk of PTLD.
*Methods: EBV+ donor lungs unsuitable for transplant were placed on EVLP alone (N = 5) or on EVLP with 500mg of RTX (N= 5) for 5-12 hours. Flow cytometry and immunohistochemistry (IHC) was used to quantify the proportion of CD19+ or CD20+ B cells in lung tissue and lymph node (LN) biopsies collected pre- and post-perfusion. EBV viral burden was assessed flow cytometric analysis of latent membrane protein 2A (LMP2A). Cell death was quantified using the TUNEL assay. Perfusate was collected to measure proinflammatory cytokines indicative of tissue damage including: IFNγ, IL-1β, TNFα, Il-6, and IL-8. To confirm successful RTX delivery, RTX levels within tissue were measured by ELISA.
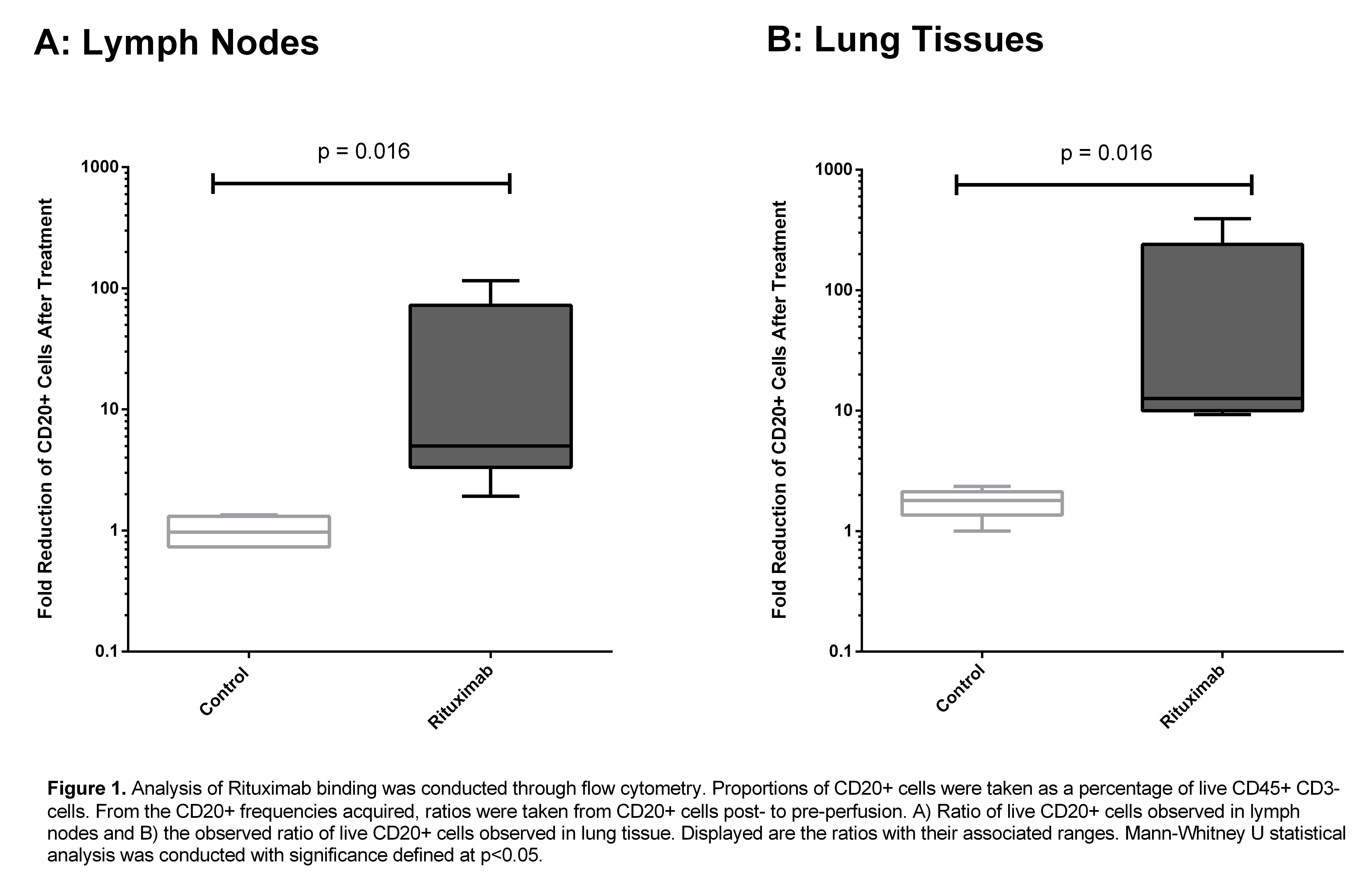
*Results: RTX was successfully delivered to lungs on EVLP with no observable physiologic adverse effects. The increase in ratio of pre-perfusion to post-perfusion live CD20+ cells was used to assess the fold reduction of B-cells after RTX treatment Lung tissue perfused with RTX showed a significant decrease in CD20+ cell ratio compared to controls (median fold-decrease 12.7 vs 1.72, p = 0.016) . A similar decrease was observed in treated LNs vs control (median fold-decrease 5.0 vs 1.0, p = 0.016).
This difference was not seen in CD19+ B-cells. IHC results were concordant with these findings. This marked decrease in CD20+ B-cell frequency was apparent as early as 5 hours post-RTX administration. Viral measurements of LMP2A revealed no significant changes after RTX perfusion. The TUNEL assay did not illustrate any significant change in cell death after RTX EVLP. ELISA demonstrated the presence of RTX in lung tissue. Preliminary cytokine profiles show no differences between groups suggesting the approach is comparably as safe as conventional EVLP.
*Conclusions: We show efficient delivery and binding of RTX to CD20+ B-cells after EVLP mediated administration with no observable harmful effects. However, the study timeframe is likely too short to show B-cell death. This study provides evidence that EVLP may effectively serve as a platform for pre-transplant graft conditioning of lungs to potentially attenuate transmission of latent viruses.
To cite this abstract in AMA style:
Ku T, Cypel M, Ribeiro RV, Ferreira VH, Kumar D, Humar A. Ex-Vivo Perfusion Mediated Delivery of Rituximab to Clear Latent Epstein-Barr Virus [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/ex-vivo-perfusion-mediated-delivery-of-rituximab-to-clear-latent-epstein-barr-virus/. Accessed February 18, 2026.« Back to 2019 American Transplant Congress