Early Use of Everolimus Plus Reduced Tacrolimus vs Standard Tacrolimus in De Novo Livertransplant Recipients: 12 Months Efficacy and Safety Data from HEPHAISTOS Study
1Study Group, Hephaistos, Germany, 2Novartis Pharma GmbH, Nuernberg, Germany
Meeting: 2019 American Transplant Congress
Abstract number: 40
Keywords: Efficacy, Immunosuppression, Liver transplantation
Session Information
Session Name: Concurrent Session: Liver: Immunosuppression and Rejection
Session Type: Concurrent Session
Date: Sunday, June 2, 2019
Session Time: 2:30pm-4:00pm
 Presentation Time: 2:30pm-2:42pm
Presentation Time: 2:30pm-2:42pm
Location: Room 312
*Purpose: The HEPHAISTOS study was set up to compare early use of everolimus [EVR] combined with reduced tacrolimus [rTAC] versus standard-dose tacrolimus [TAC-C) in de novo liver transplant [LTx] recipients
*Methods: In this 12 months [M] prospective, open-label, multi-center study 333 patients [pts] were randomized 1:1 between day 7 to 21 after Tx to either EVR(3-8ng/ml) + rTAC(<5ng/ml), or TAC-C(6-10ng/ml), all with steroids until M6. Here we report M12 efficacy and safety results (169 EVR+rTAC, 164 TAC-C).
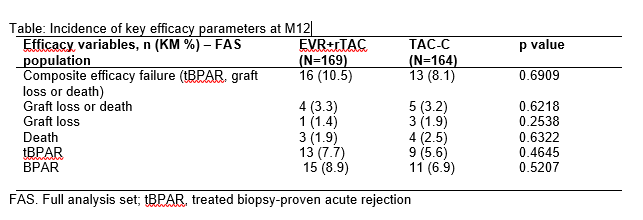
*Results: Randomization occurred on average 15 days after LTx. Efficacy between both groups was comparable. Incidence rates for Biopsy Proved Acute Rejection [BPAR] were 8.9% in EVR+rTAC and 6.9% in TAC-C group at M12 (Kaplan-Meier estimates). Most events were graded as mild (9 vs.7) and only few events as moderate or severe (in total 8 vs 7). Under treatment, no graft loss and 2 deaths occurred in the EVR arm, and 3 graft losses and 3 deaths in the TAC-C arm. Safety profiles were similar, incidences of AEs leading to study drug discontinuation were 23.7% in EVR+rTAC, vs 23.2% in TAC-C arm. Main reasons for discontinuation were renal and urinary disorders (1.2% EVR+rTAC, 7.3%TAC-C) and leucopenia (2.4%, 0.0%). No new safety signals were identified during the study
*Conclusions: HEPHAISTOS confirmed early use of EVR plus rTAC in LTx recipients is feasible and safe with good efficacy Outcomes.
To cite this abstract in AMA style:
Nashan B, Schemmer P, Schlitt H, Pascher A, Klein C, Neumann U, Kroeger I, Wimmer P, Braun F. Early Use of Everolimus Plus Reduced Tacrolimus vs Standard Tacrolimus in De Novo Livertransplant Recipients: 12 Months Efficacy and Safety Data from HEPHAISTOS Study [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/early-use-of-everolimus-plus-reduced-tacrolimus-vs-standard-tacrolimus-in-de-novo-livertransplant-recipients-12-months-efficacy-and-safety-data-from-hephaistos-study/. Accessed February 9, 2026.« Back to 2019 American Transplant Congress