Effect of Direct-Acting Hepatitis C Treatment Regimens on Calcineurin Inhibitors in Liver and Kidney Transplant Patients
M. Laub, J. Byrns, M. Harris, C. Berg, S. Sanoff.
Duke University Hospital, Durham, NC.
Meeting: 2018 American Transplant Congress
Abstract number: D245
Keywords: Calcineurin, Drug interaction, Hepatitis C, Viral therapy
Session Information
Session Name: Poster Session D: Liver: Viral Hepatitis
Session Type: Poster Session
Date: Tuesday, June 5, 2018
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall 4EF
Background: It has been suggested that treatment with direct-acting antivirals (DAA) for hepatitis C virus (HCV) may decrease calcineurin inhibitor (CNI) concentrations. The purpose of this study is to determine the effects of HCV treatment with DAA regimens on CNI troughs and doses in liver and kidney transplant recipients.
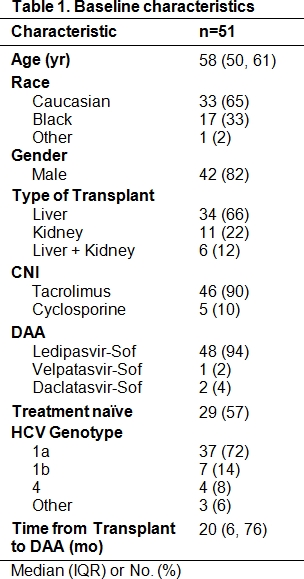
Methods: Retrospective, single center study of 51 liver and kidney transplant recipients treated for HCV between 10/2014-3/2017 with one of the following agents + ribavirin: ledipasvir-sofosbuvir, daclatasvir-sofosbuvir, velpatasvir-sofosbuvir, or elbasvir-grazoprevir. The primary outcome was percent change in CNI troughs and total daily doses (TDD) between the week prior to DAA treatment, days 21-35 during treatment, and days 21-35 following treatment completion. Secondary outcomes included sustained virologic response (SVR) and biopsy-proven acute rejection (BPAR) rates.
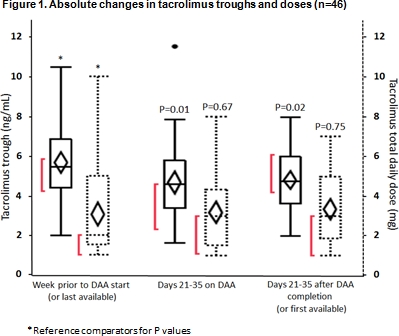
Results: The median percent difference in all CNI troughs from pre- to during DAA therapy was -20.5% (IQR -36.2, 13.1) and from during to post-DAA therapy was 9.6% (IQR -15, 35%); corresponding median percent changes in all CNI doses were 0% (IQR 0, 0%) and 0% (IQR 0, 20%), respectively. Subjects on tacrolimus experienced statistically significant changes in troughs but not doses (Figure 1). These results did not differ based on transplant type, DAA, or time from transplant. In total, 65% of subjects did not require a dose change by week 5 of DAA, 23% underwent a dose increase, and 12% a dose decrease. SVR rate was 98% and BPAR rate was 0%.
Conclusion: CNI troughs may be decreased by HCV treatment with DAA agents, but this was not associated with clinically different dosing requirements or rejection rates. Troughs and doses remained similar after completing HCV treatment, suggesting changes may be partially attributable to improved hepatic function.
CITATION INFORMATION: Laub M., Byrns J., Harris M., Berg C., Sanoff S. Effect of Direct-Acting Hepatitis C Treatment Regimens on Calcineurin Inhibitors in Liver and Kidney Transplant Patients Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Laub M, Byrns J, Harris M, Berg C, Sanoff S. Effect of Direct-Acting Hepatitis C Treatment Regimens on Calcineurin Inhibitors in Liver and Kidney Transplant Patients [abstract]. https://atcmeetingabstracts.com/abstract/effect-of-direct-acting-hepatitis-c-treatment-regimens-on-calcineurin-inhibitors-in-liver-and-kidney-transplant-patients/. Accessed February 20, 2026.« Back to 2018 American Transplant Congress