Sirolimus-Based, Prednisone-Free Regimen in Kidney Transplantation: Long Term Results of an Open-Label, Randomized Controlled Trial
1Northwestern University, Chicago
2University of Virginia, Charlottesville
3Advocate Christ Medical Center, Chicago.
Meeting: 2018 American Transplant Congress
Abstract number: C68
Keywords: Graft function, Graft survival, Immunosuppression
Session Information
Session Name: Poster Session C: Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Type: Poster Session
Date: Monday, June 4, 2018
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall 4EF
Background: Calcineurin (CNI) nephrotoxicity is implicated in chronic allograft dysfunction and presents an argument for CNI minimizing immunosuppression. We assessed the long-term effects of CNI conversion to an mTOR inhibitor in patients receiving a prednisone-free maintenance immunosuppression.
Methods: In this single center study, 275 kidney transplant recipients were induced with alemtuzumab and placed on a prednisone free maintenance immunosuppression with tacrolimus (Tac) and mycophenolate mofetil (MMF). 12 months after transplantation, patients were randomized in a 2:1 ratio to undergo CNI elimination (sirolimus-based regimen with MMF) or continue standard Tac based treatment. Patients with a recent (<3 months) acute rejection or more than 0.5g/day of proteinuria were excluded. Intention to treat analysis was performed.
Results: There were no statistical differences in recipient demographics. Mean follow time was 45.8 months in the SRL group and 46.9 months in the Tac group. Graft survival and patient survival were similar between the 2 groups. Rejection free survival 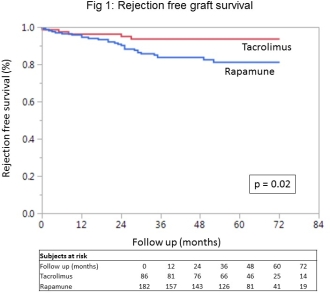 was higher in Tac group (HR 2.88, CI 1.2 – 8.5, p 0.02). However, the mTOR group had a significantly higher MDRD eGFR (4.62 ml/min/1.73 m2, CI 0.33 – 8.91, p 0.036), estimated by linear mixed effects model over 84 months of follow up.
was higher in Tac group (HR 2.88, CI 1.2 – 8.5, p 0.02). However, the mTOR group had a significantly higher MDRD eGFR (4.62 ml/min/1.73 m2, CI 0.33 – 8.91, p 0.036), estimated by linear mixed effects model over 84 months of follow up. 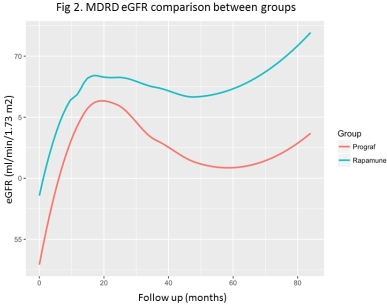
Conclusion: When compared to CNI based immunosuppression, conversion to an mTOR based regimen poses a higher risk of rejection but provides higher eGFR without difference in graft or patient survival. Future research identifying patients who do well on mTOR inhibitors could help individualize immunosuppressive therapy post kidney transplantation.
CITATION INFORMATION: Shetty A., Ho B., Mas V., Kassis Y., Park S., Leventhal J., Chhabra D., Gallon L. Sirolimus-Based, Prednisone-Free Regimen in Kidney Transplantation: Long Term Results of an Open-Label, Randomized Controlled Trial Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Shetty A, Ho B, Mas V, Kassis Y, Park S, Leventhal J, Chhabra D, Gallon L. Sirolimus-Based, Prednisone-Free Regimen in Kidney Transplantation: Long Term Results of an Open-Label, Randomized Controlled Trial [abstract]. https://atcmeetingabstracts.com/abstract/sirolimus-based-prednisone-free-regimen-in-kidney-transplantation-long-term-results-of-an-open-label-randomized-controlled-trial/. Accessed February 1, 2026.« Back to 2018 American Transplant Congress
