Successful Patency in Blood Conduits for an Artificial Kidney Membrane with Biocompatible Coatings
1VUMC, Nashville
2UCSF, San Francisco.
Meeting: 2018 American Transplant Congress
Abstract number: B390
Keywords: Bioengineering, Kidney, Preclinical trails, Xenotransplantation
Session Information
Session Name: Poster Session B: Xenotransplantation
Session Type: Poster Session
Date: Sunday, June 3, 2018
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall 4EF
BACKGROUND: An implantable artificial kidney may address the growing organ shortage and improve outcomes and quality of life for those with end stage renal disease. We have previously reported that computational modeling and in vitro imaging facilitated rapid iterative development of a low-thrombosis blood conduit. Filtration membranes must have biocompatible coatings while allowing continued patency of the conduit which we had not yet explored in vivo.
METHODS: We utilized polycarbonate hemofilter cartridges with a helical inflow design and a tapering, non-anti-parallel outflow. We used the following coatings on membrane blanks: 1) alumina 2) titanium nitride 3) polysilicon with polyethylene glycol (PEG) and 4) silicon dioxide with PEG. Each was attached to a hemofilter cartridge. We implanted these into four Class A dogs for 30 days without maintenance warfarin or heparin.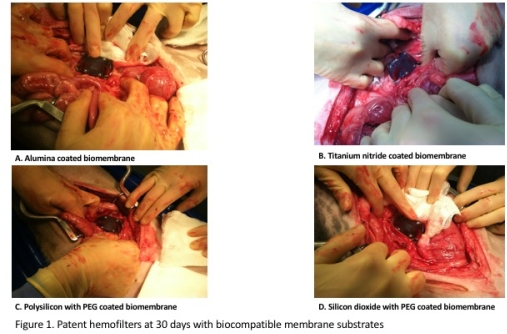 Anastomosis of the grafts for outflow and inflow to the device were performed to the abdominal IVC and aorta, respectively. Doppler ultrasound examination verified patency of blood conduits over the 30 days. At postoperative day 30, the devices were harvested and examined for thrombosis.
Anastomosis of the grafts for outflow and inflow to the device were performed to the abdominal IVC and aorta, respectively. Doppler ultrasound examination verified patency of blood conduits over the 30 days. At postoperative day 30, the devices were harvested and examined for thrombosis.
RESULTS: Conduit designs previously optimized to reduce wall shear stress were utilized in these four dogs with patency confirmed in all; ten blood conduits have now remained patent for 30 days. The animals suffered no complications of surgery. No evidence of gross hemolysis or distal embolization were noted in vivo or by laboratory values. The optically transparent membrane blanks at explant had no visible thrombosis.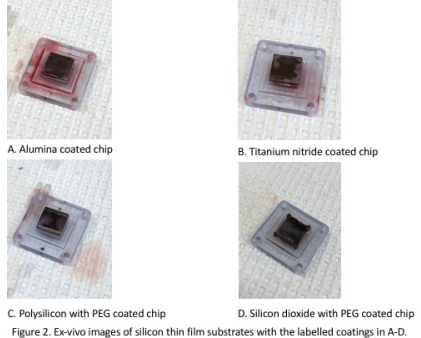
CONCLUSION: Coatings tested appear to be biocompatible and do not adversely affect patency of the blood conduit. Further investigations of the membranes will include spectroscopy and microscopy.
CITATION INFORMATION: Forbes R., Marsh E., Karp S., Buck A., Colvin D., Williams P., Chaudhuri B., Roy S., Fissell W. Successful Patency in Blood Conduits for an Artificial Kidney Membrane with Biocompatible Coatings Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Forbes R, Marsh E, Karp S, Buck A, Colvin D, Williams P, Chaudhuri B, Roy S, Fissell W. Successful Patency in Blood Conduits for an Artificial Kidney Membrane with Biocompatible Coatings [abstract]. https://atcmeetingabstracts.com/abstract/successful-patency-in-blood-conduits-for-an-artificial-kidney-membrane-with-biocompatible-coatings/. Accessed January 31, 2026.« Back to 2018 American Transplant Congress
