Efficacy and Safety of Acute Antibody Rejection Treatment in Pediatric Kidney Transplant Recipients
1University Health System, San Antonio, TX
2College of Pharmacy, Pharmacotherapy Division, The University of Texas at Austin, San Antonio, TX
3UT Health San Antonio, San Antonio, TX.
Meeting: 2018 American Transplant Congress
Abstract number: B249
Keywords: Graft function, Infection, IVIG, Plasmapheresis
Session Information
Session Name: Poster Session B: Kidney: Pediatrics
Session Type: Poster Session
Date: Sunday, June 3, 2018
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall 4EF
Purpose: Evaluate the efficacy and safety of acute antibody mediated rejection (AMR) treatment modalities in pediatric kidney transplant recipients (KTR).
Methods: A single-center retrospective chart review of pediatric KTR who received a kidney transplant between 5/1/13 to 9/30/17 was conducted. Patients < 18 years old at the time of transplant experiencing first episode of acute AMR and treated with immune globulin (IVIG), plasmapheresis (PP), corticosteroids, rituximab, and/or rabbit anti-thymocyte globulin (rATG) were included. Endpoints were collected for > 3 months from acute AMR treatment and up to 12 months, if available.
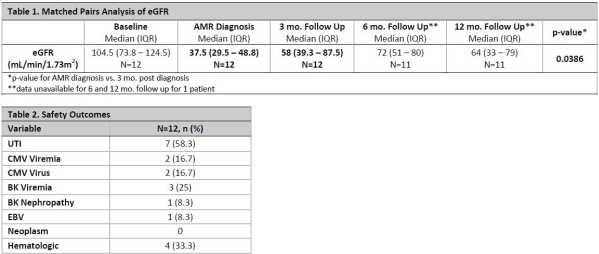
Results: 12 pediatric KTR were included that were 13.3 (±5.7) years old, 50% male, 9.5 (IQR 5.3 – 24.3) mo. since transplant, 10/12 (83%) received basiliximab vs. rATG for induction, and a median tacrolimus trough of 6.5 (IQR 4.9 – 8.8) ng/mL was found at diagnosis of acute AMR. 79 pediatric kidney transplants occurred during this timeframe. 10/12 (83%) received rituximab, 6/12 (50%) received rATG and all received IVIG and PP for rejection treatment. Patients received cumulative IVIG dose of 1916.7 (IQR 1174 – 3205) mg/kg and 5.5 (IQR 5 – 7.75) PP sessions. Histologic findings included 9/12 (75%) C4d deposits, 9/12 (75%) fibrosis and 10/12 (83)% mixed T-cell rejection. Renal function reported in Table 1. 2.3 (±2.8) total infections per patient occurred. Refer to Table 2 for all adverse events. There was no incidence of death or graft loss.
Conclusion: Findings suggest majority of patients received rituximab in addition to IVIG, PP and corticosteroids for the treatment of acute AMR, which led to a significant improvement in eGFR from the time of diagnosis to 3 mo. follow up. Reassuringly, improvement in renal function was maintained from 3- vs. 12-mo. post AMR diagnosis. AMR treatment was relatively safe with the most common AE being UTIs and hematologic effects.
CITATION INFORMATION: Kincaide E., Hitchman K., Hall R., Yamaguchi I., Crowther B. Efficacy and Safety of Acute Antibody Rejection Treatment in Pediatric Kidney Transplant Recipients Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Kincaide E, Hitchman K, Hall R, Yamaguchi I, Crowther B. Efficacy and Safety of Acute Antibody Rejection Treatment in Pediatric Kidney Transplant Recipients [abstract]. https://atcmeetingabstracts.com/abstract/efficacy-and-safety-of-acute-antibody-rejection-treatment-in-pediatric-kidney-transplant-recipients/. Accessed February 16, 2026.« Back to 2018 American Transplant Congress