Anti-Thymocyte Globulin vs. Alemtuzumab: A Comparison of Long-Term Outcomes among Renal Transplant Recipients
Department of Pharmacy, The University of Alabama at Birmingham, Birmingham, AL.
Meeting: 2018 American Transplant Congress
Abstract number: B142
Keywords: Efficacy, Induction therapy, Kidney transplantation, Safety
Session Information
Session Name: Poster Session B: Kidney Immunosuppression: Induction Therapy
Session Type: Poster Session
Date: Sunday, June 3, 2018
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall 4EF
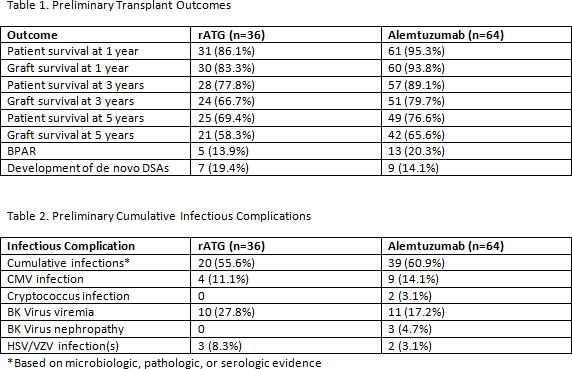
Background: Two lymphocyte depleting agents are currently available for renal transplant induction: alemtuzumab (C1H) and rabbit anti-thymocyte globulin (rATG). Studies comparing long-term outcomes between C1H and rATG beyond 3 years post-transplant have been limited. Here we compare the efficacy and safety of rATG and C1H at 1, 3, and 5 years post-renal transplant.
Methods: The current study is a single-center, retrospective analysis of renal transplant recipients who received C1H (30 mg intraoperatively) or rATG (4.5-6 mg/kg total dose) for induction. Patient (PS) and graft survival (GS), episodes of biopsy proven acute rejection (BPAR), rates of infection, and rates of leukopenia were compared at 1, 3, and 5 years post-transplant.
Results: Patient demographics were similar between the rATG (n=36) and C1H groups (n=64). Patients were primarily recipients of a deceased donor transplant (60%), and patients in the rATG group had a higher panel reactive antibody (35 vs. 8%) prior to transplant. PS and GS were similar at 5 years (69.4 vs. 76.6%; 58.3 vs. 65.6%, respectively), as well as rates of BPAR (13.9 vs 20.3%). Incidence of infection was similar between groups (55.6 vs. 60.9%), although rates of leukopenia were higher in the C1H group (25 vs. 57.8%) with more need for granulocyte-colony stimulating factor (GCSF) (2.8 vs. 10.9%). Formal statistical analysis is pending upon completion of data collection.
Conclusions: Preliminary results suggest similar efficacy between C1H and rATG in terms of PS, GS, and BPAR at 5 years post-transplant. Infectious complications were comparable, while hematologic toxicity was greater in the C1H group, including greater GCSF use. Both C1H and rATG appear to be effective induction agents in renal transplantation, although use of C1H may be associated with higher incidence of myelosuppression.
CITATION INFORMATION: Loncharic E., Dodson A., Gutierrez K. Anti-Thymocyte Globulin vs. Alemtuzumab: A Comparison of Long-Term Outcomes among Renal Transplant Recipients Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Loncharic E, Dodson A, Gutierrez K. Anti-Thymocyte Globulin vs. Alemtuzumab: A Comparison of Long-Term Outcomes among Renal Transplant Recipients [abstract]. https://atcmeetingabstracts.com/abstract/anti-thymocyte-globulin-vs-alemtuzumab-a-comparison-of-long-term-outcomes-among-renal-transplant-recipients/. Accessed January 7, 2026.« Back to 2018 American Transplant Congress