Influence of CYP3A5 and ABCB1 Genotypes on Pharmacokinetics of Immediate and Prolonged Release Tacrolimus Preparations
1Clinical Pharmacology Department, Queen Mary University of London, London, United Kingdom
2Analytical Services International, St.George's University of London, London, United Kingdom
3Institute of Medical and Biomedical Education-Renal Medicine, St.George's University of London, London, United Kingdom.
Meeting: 2015 American Transplant Congress
Abstract number: D122
Keywords: FK506, Gene polymorphism, Kidney transplantation, P-glycoprotein
Session Information
Session Name: Poster Session D: Kidney Immunosuppression: Drug Minimization
Session Type: Poster Session
Date: Tuesday, May 5, 2015
Session Time: 5:30pm-6:30pm
 Presentation Time: 5:30pm-6:30pm
Presentation Time: 5:30pm-6:30pm
Location: Exhibit Hall E
Background. Tacrolimus is available in two formulations, immediate-release, (Prograf®) and prolonged -release formulation of tacrolimus (Advagraf®). Tacrolimus has a narrow therapeutic index with wide variation between- and within individuals. It is mainly metabolized by CYP3A4/5 and is transported by P-glycoprotein (P-gp). Expression of CYP3A decreases and expression of P-gp increases along the length of the gut.
Objective. We aimed to determine whether the CYP3A5*3 and ABCB1 genotypes influence the pharmacokinetics of prolonged-release tacrolimus in the same way as is well established for the immediate release preparation, in stable renal transplant recipients.
Methods. A total of sixty-four stable renal transplant recipients treated with twice daily tacrolimus (Prograf®) were switched to the same total daily dose of Advagraf® with 24 hour pharmacokinetic profiles before and two weeks after the change. Patients were divided into 4 genotype categories based on expression of CYP3A5 (*1/*1 or *1/*3), CYP3A5 non-expressers (*3/*3), high expressers of P-gp (ABCB1; CC) or low expressers of P-gp (ABCB1; CT or TT).
Results. ABCB1 polymorphisms contributed to significant changes in tacrolimus pharmacokinetic parameters and dose requirements only in CYP3A5*1 allele carriers. Dose-normalized pharmacokinetic parameters were significantly lower in CYP3A5 expressers than in CYP3A5 non-expressers for both preparations (Table). The influence of the CYP3A5 and ABCB1 genotype on tacrolimus exposure was the same for the prolonged release preparation Advagraf® as for the immediate release preparation, Prograf®.
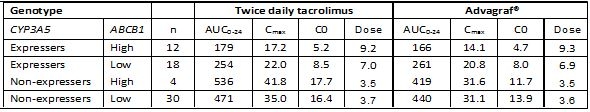
Conclusion. Pharmacogenetic dosing strategies based on these genotypes are likely to be equally applicable to prescribing the once daily tacrolimus formulation, Advagraf®, as to twice daily formulations.
To cite this abstract in AMA style:
Elnahhas T, Lee T, Moreton M, McKeown D, Popoola J, Ramkhelawon R, Johnston A, MacPhee I. Influence of CYP3A5 and ABCB1 Genotypes on Pharmacokinetics of Immediate and Prolonged Release Tacrolimus Preparations [abstract]. Am J Transplant. 2015; 15 (suppl 3). https://atcmeetingabstracts.com/abstract/influence-of-cyp3a5-and-abcb1-genotypes-on-pharmacokinetics-of-immediate-and-prolonged-release-tacrolimus-preparations/. Accessed February 17, 2026.« Back to 2015 American Transplant Congress
