Normothermic Machine Perfusion (NMP) Preservation for Human Liver Transplantation in the Cleveland Clinic
Cleveland Clinic, Cleveland, OH.
Meeting: 2018 American Transplant Congress
Abstract number: 199
Keywords: Liver transplantation, Machine preservation
Session Information
Session Name: Concurrent Session: Ischemia Reperfusion Injury: Time to Change the Paradigm?
Session Type: Concurrent Session
Date: Monday, June 4, 2018
Session Time: 2:30pm-4:00pm
 Presentation Time: 2:54pm-3:06pm
Presentation Time: 2:54pm-3:06pm
Location: Room 6B
NMP is a novel preservation method for liver grafts. We transplanted 15 human livers after NMP to test the safety and feasibility of NMP on a device developed in our institution. Livers included 10 from donors after brain death (DBD) and 5 after circulatory death (DCD). Cold ischemia time before NMP was 1hrs32mins to 3hrs59mins. NMP time was 3hrs20mins to 7hrs52mins. Livers were perfused through portal vein and hepatic artery in physiologic flows and pressures with perfusate based on human red blood cells and fresh frozen plasma. During NMP, bile production was 3-13 ml/hr of DBD livers and 1-6 ml/hr of DCD livers. All livers displayed lactate clearance.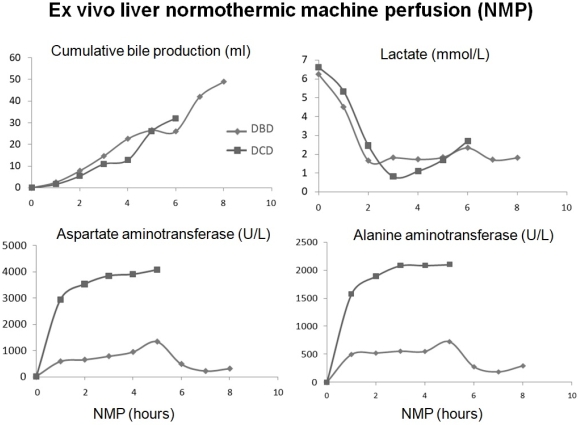 After transplantation, the early allograft dysfunction (EAD) ratio was 26.7% of NMP group and 43.3% (p=0.43) of the controls, preserved by cold storage matched (1:4) by age, donor risk index, MELD score, total preservation time. NMP group had lower peak alanine aminotransferase (p=0.001) and total bilirubin at post-operative day (POD) -7 (p=0.03). The length of stay (LOS) in ICU was 2.3±1.7 days of NMP group and 4.6±7.7 days of the controls (p=0.03). The LOS in hospital was 12.3±9.4 and 15.9±14.5 days respectively (p=0.25). The aminotransferases in perfusate at the end of NMP had significant correlation (p=0.001) to their peak values in the first 7 PODs.
After transplantation, the early allograft dysfunction (EAD) ratio was 26.7% of NMP group and 43.3% (p=0.43) of the controls, preserved by cold storage matched (1:4) by age, donor risk index, MELD score, total preservation time. NMP group had lower peak alanine aminotransferase (p=0.001) and total bilirubin at post-operative day (POD) -7 (p=0.03). The length of stay (LOS) in ICU was 2.3±1.7 days of NMP group and 4.6±7.7 days of the controls (p=0.03). The LOS in hospital was 12.3±9.4 and 15.9±14.5 days respectively (p=0.25). The aminotransferases in perfusate at the end of NMP had significant correlation (p=0.001) to their peak values in the first 7 PODs.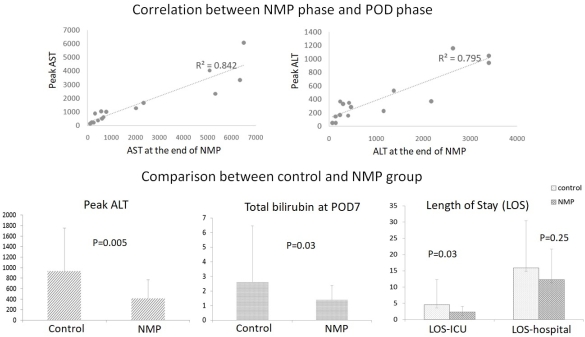 All NMP patients and grafts survived to dates (post-operative 7-18 months). Three cases (2 DCD, 1 DBD) had ERCP and stent to treat biliary stricture, comparable to the controls. These results indicated the safety and feasibility of our NMP preservation and potential benefits to protect and predict liver viability.
All NMP patients and grafts survived to dates (post-operative 7-18 months). Three cases (2 DCD, 1 DBD) had ERCP and stent to treat biliary stricture, comparable to the controls. These results indicated the safety and feasibility of our NMP preservation and potential benefits to protect and predict liver viability.
CITATION INFORMATION: Liu Q., Pezzati D., Hassan A., Soliman B., Grady P., Maikhor S., Iuppa G., Diago Uso T., Hashimoto K., Aucejo F., Fujiki M., Eghtesad B., Cywinski J., Irefin S., Fung J., Abu-Elmagd K., Miller C., Quintini C. Normothermic Machine Perfusion (NMP) Preservation for Human Liver Transplantation in the Cleveland Clinic Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Liu Q, Pezzati D, Hassan A, Soliman B, Grady P, Maikhor S, Iuppa G, Uso TDiago, Hashimoto K, Aucejo F, Fujiki M, Eghtesad B, Cywinski J, Irefin S, Fung J, Abu-Elmagd K, Miller C, Quintini C. Normothermic Machine Perfusion (NMP) Preservation for Human Liver Transplantation in the Cleveland Clinic [abstract]. https://atcmeetingabstracts.com/abstract/normothermic-machine-perfusion-nmp-preservation-for-human-liver-transplantation-in-the-cleveland-clinic/. Accessed February 17, 2026.« Back to 2018 American Transplant Congress
