Prospective DSA Monitoring and Significance of De Novo DSA in Adult Liver Transplantation.
1Department of Surgery, Division of Transplantation, Indiana University School of Medicine, Indianapolis, IN
2Department of Pathology, Indiana University School of Medicine, Indianapolis, IN
3Department of Gastroenterology, Division of Hepatology, Indiana University School of Medicine, Indianapolis, IN
4Department of Medicine, Immunology/Histocompatibility Laboratory, Indiana University School of Medicine, Indianapolis, IN
Meeting: 2017 American Transplant Congress
Abstract number: D201
Keywords: Antibodies, Biopsy, Liver
Session Information
Session Name: Poster Session D: Liver: Immunosuppression and Rejection
Session Type: Poster Session
Date: Tuesday, May 2, 2017
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall D1
Background: Post transplant course of circulating de novo donor specific antibodies (DSA) remains unknown after liver transplantation (LT).
Methods: In a prospective study, sera from 92 adult LT recipients were prospectively screened for DSA by Luminex Single antigen test at 1,2,3, 6,12,18,24 & 36 months post-transplant. DSA with MFI >5000 were considered significant. Standard immunosuppression included thymoglobulin-rituximab induction & tacrolimus maintenance.
Results: Seventeen (18%) patients developed de novo DSA at a median post-transplant period of 1(1-18) months, and reached MFI >5000 at median period of 12(1-24) months post-transplant. 11/92 patients had pre-formed DSA, of these one patients developed de novo DSA to new alleles. Class-II DSA were observed (94%) predominantly. Mild acute cellular rejection was observed in 2/18 patients with de novo DSA at 6 and 36 months post-transplant with cumulative MFIs 74,400 & 22,300 respectively. At a median follow up of 5.8(1.5-6.6) years 15/18 patients with de novo DSA have excellent allograft function. Two patients developed HCV recurrence and one patient died from HCC. 53 biopsies were performed during post-transplant course in patients with de novo DSA. Chronic rejection was not found in any of the biopsies. Graft loss was not significantly influenced by presence of de novo DSA. No recipient factors correlated with an increased risk of de novo DSA formation.
Conclusions: Formation of de novo DSA is common in LT recipients. With potent induction immunosuppression de novo DSA formation is without apparent clinical significance. Routine monitoring for DSA may be limited to high risk patients.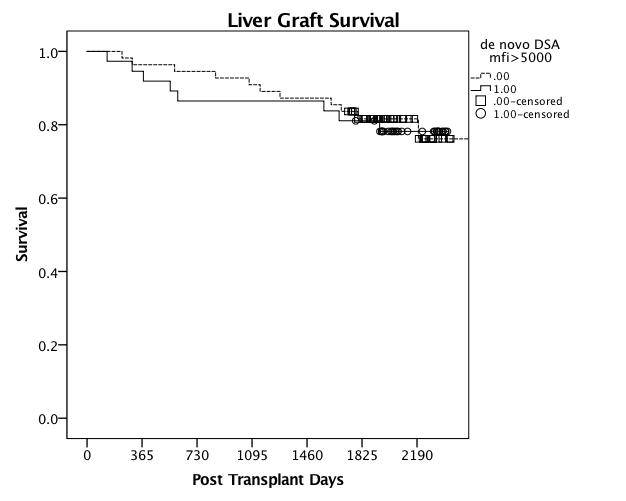
CITATION INFORMATION: Kubal S, Mangus R, Fridell J, Ekser B, Saxena R, Rush N, Higgins N, Ghabril M, Lacerda M, Lobashevsky A. Prospective DSA Monitoring and Significance of De Novo DSA in Adult Liver Transplantation. Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Kubal S, Mangus R, Fridell J, Ekser B, Saxena R, Rush N, Higgins N, Ghabril M, Lacerda M, Lobashevsky A. Prospective DSA Monitoring and Significance of De Novo DSA in Adult Liver Transplantation. [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/prospective-dsa-monitoring-and-significance-of-de-novo-dsa-in-adult-liver-transplantation/. Accessed March 3, 2026.« Back to 2017 American Transplant Congress
