Mitochondrial Dysfunction Is a Common Feature of Injury in Donor Kidneys After Brain and Circulatory Death.
1University of Oxford, Oxford, United Kingdom
2University of Groningen, Groningen, Netherlands
3University of Sydney, Sydney, Australia
Meeting: 2017 American Transplant Congress
Abstract number: D51
Keywords: Donation, Ischemia, Reactive oxygen species
Session Information
Session Name: Poster Session D: Ischemic Injury and Organ Preservation Session III
Session Type: Poster Session
Date: Tuesday, May 2, 2017
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall D1
The pro-coagulatory and pro-inflammatory environment in donors after brain death (DBD) and warm ischemia in donors after circulatory death (DCD) can cause injury to target organs, such as kidneys, compromising their quality prior to transplantation and affecting patient outcomes. Understanding how kidneys are injured by these processes will allow to develop novel protective strategies in the donor in order to improve outcomes post-transplantation, especially in the case of high risk donors.
In both pre-clinical Brain Death (BD) and Ischemia-Reperfusion Injury (IRI) models (4h BD in the rat and unilateral 45min renal ischemia followed by 24h reperfusion, respectively), mitochondrial function and bioenergetics were found to be altered compared to healthy controls using proteomics and metabolomics. Omics findings were further validated (by western blot, enzymatic, amperometric and luminescent assays) and highlighted disturbances in ATP, mitochondrial function and oxidative stress.
Proteomics and metabolomics results suggested extensive mitochondrial dysfunction. Mitochondria from DBD and IRI kidneys both showed decreased O2 consumption (Figure 1) (p<0.05) which correlated with decreased tissue ATP levels, compared to controls. In DBD, mitochondrial dysfunction was associated with increased inflammation (NFkB mRNA levels, p=0.01) and increased oxidative stress (p=0.01), compared to controls.
BD and IRI result in mitochondrial dysfunction and oxidative stress in the kidney, contributing to tissue injury. This renders the graft more susceptible to reperfusion injury once transplanted and potentially increases the risk of DGF. DBD and DCD donor samples from the UK QUOD biobank are currently being investigated to establish whether these phenotypes are also present in deceased human donors. Mitochondrial protective strategies are now possible interventions and may reduce injury to donor organs improving outcomes after transplantation.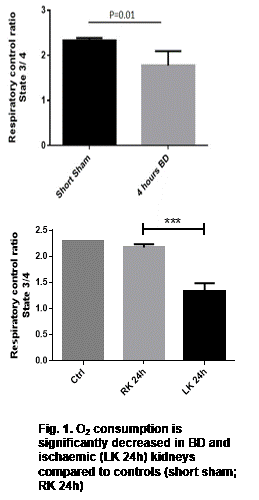
CITATION INFORMATION: Lo Faro M, Akhtar M, Huang H, Kaisar M, Rebolledo R, Morten K, Heather L, Dona A, Leuvenink H, Fuggle S, Kessler B, Pugh C, Ploeg R. Mitochondrial Dysfunction Is a Common Feature of Injury in Donor Kidneys After Brain and Circulatory Death. Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Faro MLo, Akhtar M, Huang H, Kaisar M, Rebolledo R, Morten K, Heather L, Dona A, Leuvenink H, Fuggle S, Kessler B, Pugh C, Ploeg R. Mitochondrial Dysfunction Is a Common Feature of Injury in Donor Kidneys After Brain and Circulatory Death. [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/mitochondrial-dysfunction-is-a-common-feature-of-injury-in-donor-kidneys-after-brain-and-circulatory-death/. Accessed February 7, 2026.« Back to 2017 American Transplant Congress
