Low Dose Valganciclovir for CMV Prophylaxis in the First Year of Kidney Transplantation.
P. Klem,1 L. Al-Omar,2 J. Cooper,2 S. Davis,2 J. Gralla,2 D. Choe,1 A. Wiseman.2
1Pharmacy, University of Colorado, Aurora, CO
2Nephrology, University of Colorado, Aurora
Meeting: 2017 American Transplant Congress
Abstract number: A174
Keywords: Cytomeglovirus, Kidney transplantation, Viral therapy
Session Information
Session Name: Poster Session A: Kidney Complications I
Session Type: Poster Session
Date: Saturday, April 29, 2017
Session Time: 5:30pm-7:30pm
 Presentation Time: 5:30pm-7:30pm
Presentation Time: 5:30pm-7:30pm
Location: Hall D1
Valganciclovir (VGC) is the standard antiviral agent used for the prevention of CMV diseases in moderate and high risk renal transplant recipients, yet the optimal dose that balances efficacy with cost and toxicity has yet to be established. The purpose of this study was to evaluate the effectiveness of low dose VCG for CMV prevention.
Methods:
From January 2012 to November 2015, kidney and kidney-pancreas recipients were initiated on VGC 450 mg daily for primary prophylaxis of CMV in the setting of maintenance immunosuppression with tacrolimus, mycophenolate, and prednisone. R-ATG was given for induction therapy to high immunologic risk patients. Duration of VGC therapy for recipients with serostatus CMV donor positive (D+) and recipient negative (R-) was 6 months, while D+/R+ and D-/R+ patients induced with r-ATG were treated for 3 months. D+/R+ and D-/R+ without r-ATG induction and D-/R- received no prophylaxis. Incidence of CMV disease was determined over a 12 month period, defined as CMV viremia with systemic systems.
Results:
522 patients were included in the analysis and CMV disease occurred in 33 patients (6.3%) at a mean of 163 days post-transplant.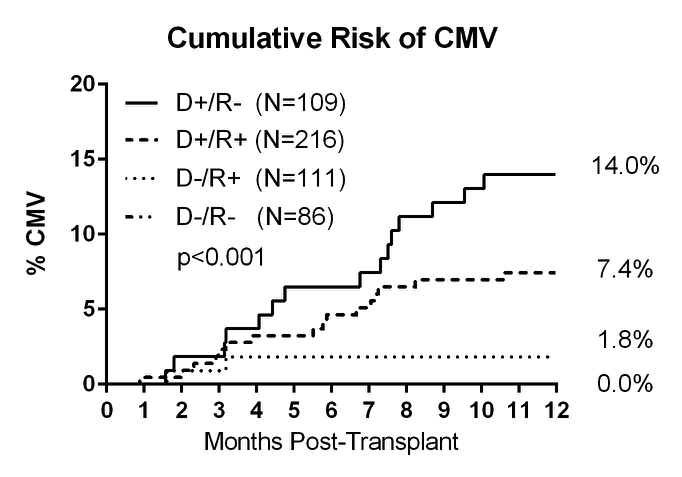 Gastritis was the most common presenting symptom (13/33, 39%). D+/R- patients had the highest risk of developing CMV disease and 5 out the 15 patients had VGC therapy held due to neutropenia prior to developing CMV disease. Additionally, 2 of the D+/R+ patients developed CMV disease after receiving r-ATG for rejection therapy, during which time VGC prophylaxis was not utilized.
Gastritis was the most common presenting symptom (13/33, 39%). D+/R- patients had the highest risk of developing CMV disease and 5 out the 15 patients had VGC therapy held due to neutropenia prior to developing CMV disease. Additionally, 2 of the D+/R+ patients developed CMV disease after receiving r-ATG for rejection therapy, during which time VGC prophylaxis was not utilized.
Conclusions
Use of low dose VGC resulted in an incidence of CMV disease of 6.3% at 12 months. This data supports the use of selective prophylaxis for moderate risk patients (D+/R+ or D-/R+ with r-ATG induction therapy) in addition to universal prophylaxis for high risk populations. Providers must be vigilant of the risks of developing CMV disease in at risk populations while holding VGC due to an adverse effect or during treatment of acute rejection with r-ATG.
CITATION INFORMATION: Klem P, Al-Omar L, Cooper J, Davis S, Gralla J, Choe D, Wiseman A. Low Dose Valganciclovir for CMV Prophylaxis in the First Year of Kidney Transplantation. Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Klem P, Al-Omar L, Cooper J, Davis S, Gralla J, Choe D, Wiseman A. Low Dose Valganciclovir for CMV Prophylaxis in the First Year of Kidney Transplantation. [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/low-dose-valganciclovir-for-cmv-prophylaxis-in-the-first-year-of-kidney-transplantation/. Accessed February 10, 2026.« Back to 2017 American Transplant Congress
