Pre-Transplant HLA-Specific B Cell Breadth of Reactivity in a Novel Multiplex Bead-Based Assay Predicts Rejection in Kidney Transplant Recipients.
1Northwestern University, Chicago
2Icahn School of Medicine at Mount Sinai, New York
Meeting: 2017 American Transplant Congress
Abstract number: 403
Keywords: B cells, HLA antibodies, Kidney transplantation, Rejection
Session Information
Session Name: Concurrent Session: Diagnosis of Antibody Mediated Rejection in Kidney Transplant Recipients
Session Type: Concurrent Session
Date: Tuesday, May 2, 2017
Session Time: 2:30pm-4:00pm
 Presentation Time: 3:42pm-3:54pm
Presentation Time: 3:42pm-3:54pm
Location: E354a
Introduction: Recipients with broad sensitization to HLA defined by a high percentage of Panel Reactive Antibodies (PRA) are at higher immunologic risk for poor graft outcomes. Little is known about the impact of breadth of circulating HLA-specific B cells (HSB) on graft outcomes. Utilizing a novel multiplex bead-based assay to identify HSB, we studied the circulating HSB in kidney transplant recipients and correlated with development of acute rejection (AR).
Methods: FlowPRA Screening Test (One Lambda) was used to detect HLA Class I and II specific antibodies and percent PRA in pre-transplant sera from kidney transplant recipients (n=12; 8 with no AR and 4 with AR) in CTOT-01 study (Hricik AJT 2013). PBMC were incubated with single antigen HLA-coated multiplexed beads (SAB) (One Lambda) for HLA-I and II. Using multi-parameter flow cytometry, HSB were identified by the formation of HLA Bead-B-cell Rosettes (BBR) (Blue dots, Figs.1C & 1D). HLA-specific B cell breadth of reactivity termed as Panel Reactive B cells (PRB) was defined as the percentage of total SAB forming BBR.
Results: Pre-transplant PRB for both class I (52.58±17.9 vs 21.13±4.7; p=0.04 Figure 1A) and II (40.53±14 vs 14.08±2.3; p=0.02 Figure 1B) was significantly higher in subjects who developed rejection. Representative flow cytometry plots from subjects with no rejection (Figure 1C) and rejection (Figure 1D). Each orange region represents different HLA specificity and each Blue dot represents a BBR. Pre-transplant HLA-I PRB (r=0.584, p=0.046) and HLA-II PRB (r=0.642, p=0.024) correlated with development of rejection. However, we found no correlation with pre-transplant PRA and development of rejection.
Conclusion: The degree of B cell reactivity against HLA, as assessed by Panel Reactive B cells (PRB) but not by PRA, for both HLA Class I and II pre-transplantation predicted rejection. These data suggest that circulating B cells specific for HLA antigens assessed by this novel assay may serve as more sensitive potential biomarker for risk stratification of kidney transplant recipients.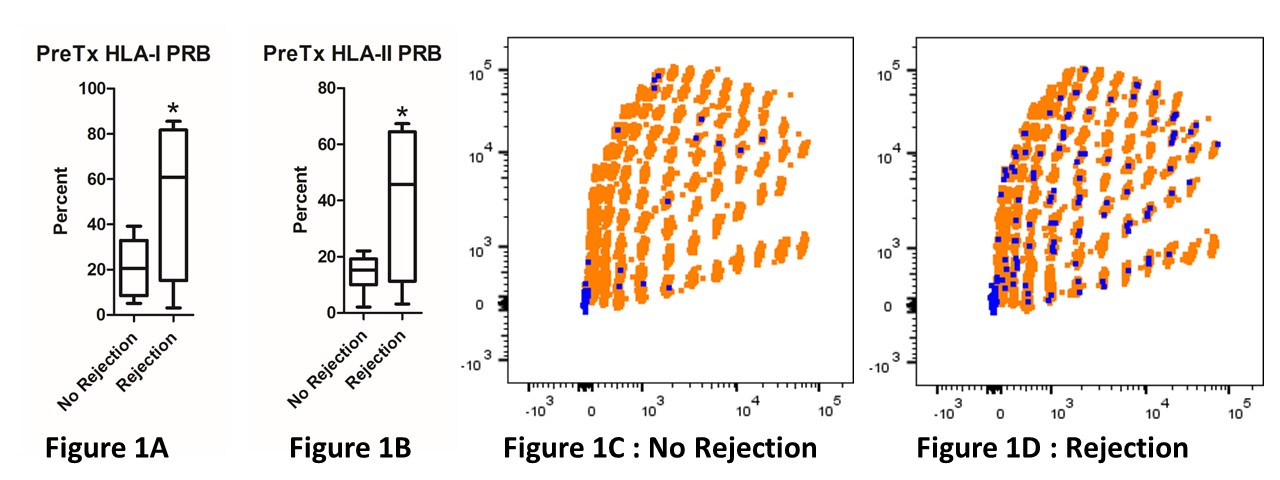
CITATION INFORMATION: Ilyas Z, Aki A, Tambur A, Heeger P, Ansari M. Pre-Transplant HLA-Specific B Cell Breadth of Reactivity in a Novel Multiplex Bead-Based Assay Predicts Rejection in Kidney Transplant Recipients. Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Ilyas Z, Aki A, Tambur A, Heeger P, Ansari M. Pre-Transplant HLA-Specific B Cell Breadth of Reactivity in a Novel Multiplex Bead-Based Assay Predicts Rejection in Kidney Transplant Recipients. [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/pre-transplant-hla-specific-b-cell-breadth-of-reactivity-in-a-novel-multiplex-bead-based-assay-predicts-rejection-in-kidney-transplant-recipients/. Accessed February 17, 2026.« Back to 2017 American Transplant Congress
