Extended Experience with Tocilizumab (Anti-IL6R) for Difficult to Desensitize (Des) Patients Awaiting HLA Incompatible Kidney Transplant.
Kidney Transplant, Cedars-Sinai Medical Center, LA, CA
Meeting: 2017 American Transplant Congress
Abstract number: 108
Keywords: Highly-sensitized, HLA antibodies, Monoclonal antibodies, Rejection
Session Information
Session Name: Concurrent Session: Clinical Science: Kidney Immunosuppression: Desensitization
Session Type: Concurrent Session
Date: Sunday, April 30, 2017
Session Time: 4:30pm-6:00pm
 Presentation Time: 5:30pm-5:42pm
Presentation Time: 5:30pm-5:42pm
Location: E354b
INTRODUCTION: ~30% of HS patients fail to respond to DES with IVIG+rituximab. IL6/IL-6R signaling is critical for B-cell differentiation to plasmablasts and IgG production. Tocilizumab (TCZ) is a 1st in-class monoclonal directed at the IL-6R. TCZ treatment in humans inhibits TFH & Th17 cells while increasing Tregs and suppressing plasmablasts. We previously reported on the efficacy of TCZ as a DES agent. Here we report our extended experience using DES with PLEX+IVIG+TCZ. PATIENTS & METHODS: From 12/7/10 – 6/29/16, 26 HS patients were DES with PLEX+IVIG+TCZ. Acceptable crossmatch criteria included: negative CDC-CMX, FCMX ≤225MCS and DSA ≤10,000MFI post-DES. Unacceptable antigens were defined as MFI>15000. Transplanted patients received induction with alemtuzumab and maintained with tac/mmf/pred + monthly TCZ x6M. Patient & graft survival, eGFR, freedom from ABMR and change in DSA levels post-TCZ were analyzed. RESULTS: Ten patients did not achieve DSA reduction sufficient for transplantation after TCZ. All had previous transplants and multiple C1Q+ HLA specificities with CPRA 99-100% {G1}. However, 16 patients were transplanted after TCZ DES {G2}. Briefly, 11 patients were FCMX+/DSA+ @transplant. Three patients developed ABMR post-transplant, but responded to PLEX/IVIG/Ritux and continuation of TCZ X 6M (Figure 1). A significant reduction in DSA from pre- to post-transplant was seen in TCZ treated patients (P<0.03) (Figure 2). Patient & graft survival up to 36M was 100%. Mean time to transplant from 1st DES was 28±17.5M. However, after TCZ,was 4±3.3M. Finally, eGFR were similar at 12M, 24M & 36M (~57±21 ml/min/1.73m2). CONCLUSIONS: 26 patients were DES with TCZ. Sixteen patients received incompatible transplants with no patient or graft loss. Three patients developed ABMR, usually after cessation of TCZ therapy. TCZ DES resulted in significant reduction of DSA post-transplant. Therefore, TCZ may offer benefit in improving rates of transplant for HS patients who fail to respond to IVIG+rituximab therapy and benefits in maintaining the allograft long-term. 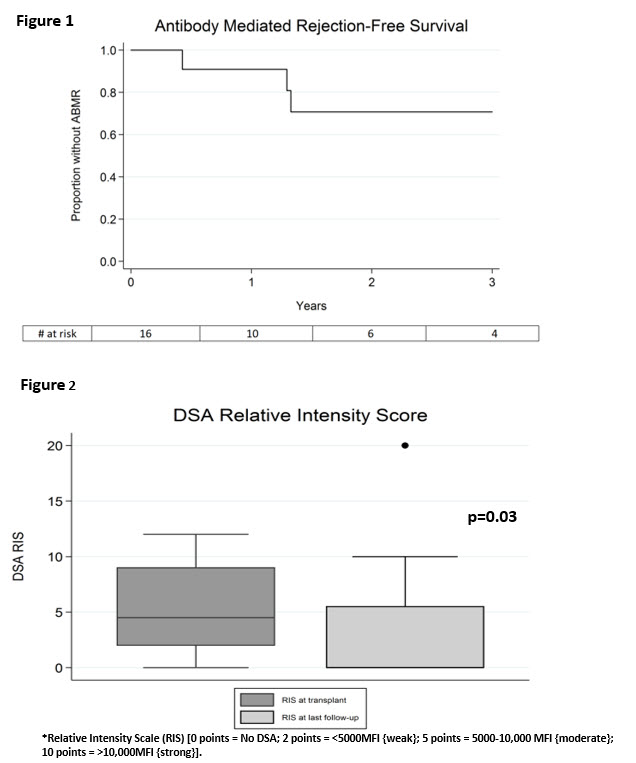 [figure2]
[figure2]
CITATION INFORMATION: Vo A, Choi J, Kim I, Huang E, Louie S, Kang A, Williamson S, Myers K, Peng A, Najjar R, Jordan S. Extended Experience with Tocilizumab (Anti-IL6R) for Difficult to Desensitize (Des) Patients Awaiting HLA Incompatible Kidney Transplant. Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Vo A, Choi J, Kim I, Huang E, Louie S, Kang A, Williamson S, Myers K, Peng A, Najjar R, Jordan S. Extended Experience with Tocilizumab (Anti-IL6R) for Difficult to Desensitize (Des) Patients Awaiting HLA Incompatible Kidney Transplant. [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/extended-experience-with-tocilizumab-anti-il6r-for-difficult-to-desensitize-des-patients-awaiting-hla-incompatible-kidney-transplant/. Accessed January 27, 2026.« Back to 2017 American Transplant Congress
