Sirolimus Regimen and Viral Serology as Risk Factors for Outcomes of Adult Kidney Transplants: 14-Year Cumulative Data in the United States.
Department of Medicine, Univ. of Florida, College of Medicine, Gainesville, FL.
Meeting: 2016 American Transplant Congress
Abstract number: 68
Keywords: Cytomeglovirus, Hepatitis B, Hepatitis C, Sirolimus (SLR)
Session Information
Session Name: Concurrent Session: Policy and Practice: Implications for Long Term Outcomes
Session Type: Concurrent Session
Date: Sunday, June 12, 2016
Session Time: 2:30pm-4:00pm
 Presentation Time: 3:42pm-3:54pm
Presentation Time: 3:42pm-3:54pm
Location: Room 312
BACKGROUND:
The relative risks for patient mortality, graft loss and malignancy in the five years following kidney-only transplant (KT) based on sirolimus (SRL) regimen at year-one and pre-transplant serology for Hepatitis B (HBV) or C (HCV), Cytomegalovirus (CMV), and Epstein Barr virus (EBV) have not been reported in a single study of a national transplant database before.
METHODS:
We retrospectively studied 72574 kidney-only transplants in US adults between 1/1/2000 and 12/31/2013 with conditional one-year graft survival. Among these; 2827 (3.9%) were on SRL+mycophenolate (MPA), 3124 (4.3%) were on SRL+Tacrolimus (Tac) and 66623 (91.8 %) were on Tac+MPA at one-year post-transplant follow-up. We used Cox multivariable models to analyze the adjusted hazard ratios (aHR) for 5-year post-transplant mortality, graft loss and malignancy related to the SRL regimens (versus reference:Tac+MPA ) and serology statuses for HBV, HCV, CMV and EBV.
RESULTS:
Compared with Tac+MPA, SRL+MPA and SRL+Tac are associated with higher risks of mortality [aHR=1.42, 95%CI=1.25-1.61 and aHR=1.57, 95% CI=1.39-1.77, respectively) and death-censored graft loss, (DCGL),(aHR=1.40, 95% CI=1.24-1.59 and aHR=1.41, 95% CI=1.26-1.58, respectively), (not shown). Post-transplant malignancy risk is significantly or suggestively reduced in relation to SRL+MPA (aHR=0.82, 95%CI=0.68-0.98) or SRL+Tac (aHR=0.88, 95% CI=0.74-1.05), respectively, (not shown). The figures below show the outcomes associated with SRL regimen and viral serology interactions: 1 increased risks of mortality with any SRL regimen vs. Tac+MPA regardless of CMV or EBV serostatus; 2. In HCV+ recipients, mortality risk is not increased with SRL+MPA and increased with SRL+Tac, compared with Tac+MPA; 3. Recipients with negative HBV, HCV, CMV and EBV serologies have lower malignancy risks with SRL+MPA vs. Tac+MPA.
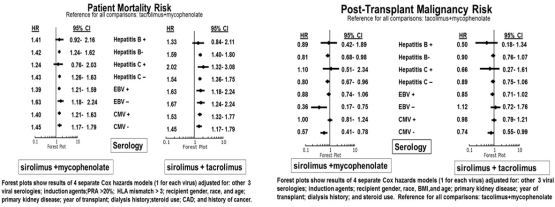
CONCLUSIONS:
SRL regimen and pre-transplant viral serologies are predictors of five-year graft loss, patient mortality and post-transplant malignancy in kidney allograft recipients with conditional one-year graft survival.
CITATION INFORMATION: Santos A, Casey M, Wen X, Rehman S, Womer K. Sirolimus Regimen and Viral Serology as Risk Factors for Outcomes of Adult Kidney Transplants: 14-Year Cumulative Data in the United States. Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Santos A, Casey M, Wen X, Rehman S, Womer K. Sirolimus Regimen and Viral Serology as Risk Factors for Outcomes of Adult Kidney Transplants: 14-Year Cumulative Data in the United States. [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/sirolimus-regimen-and-viral-serology-as-risk-factors-for-outcomes-of-adult-kidney-transplants-14-year-cumulative-data-in-the-united-states/. Accessed March 5, 2026.« Back to 2016 American Transplant Congress
