Long-Term Assessment of the Safety and Efficacy of IVIG and Rituximab-Based Desensitization (DES).
Comprehensive Transplantation Center, Cedars-Sinai Medical Center, Los Angeles, CA.
Meeting: 2016 American Transplant Congress
Abstract number: 319
Keywords: CD20, Highly-sensitized, IVIG, Kidney transplantation
Session Information
Session Name: Concurrent Session: Kidney: Desensitization
Session Type: Concurrent Session
Date: Monday, June 13, 2016
Session Time: 4:30pm-6:00pm
 Presentation Time: 4:42pm-4:54pm
Presentation Time: 4:42pm-4:54pm
Location: Room 304
Introduction: HLA sensitized patients constitute a growing number of ESRD patients with an immunologic barrier to successful transplantation. Desensitization (DES) therapies have emerged to address this problem in efforts to improve transplant rates. Here we report our 10 year experience with high dose IVIG + Rituximab DES and compare outcomes to non-sensitized (NS) pts. Patients & Methods: From 8/2005 to 8/2015, we DES 514 HS patients. HS is defined as PRA ≥ 30% (most>80%). DES includes IVIG 2g/kg (max >70kg, 140g) infusion on day 0 & day 30 and Rituximab 1gm or 375mg/m2 on day 15 +/- plasma exchange (PLEX) + pre-meds. Pts proceeded to transplant when CDC was negative (≤1:2 dilution), T and B FCMX MCS ≤225, & DSA scores acceptable. If pts were not transplanted after 6 months, repeat DES was given. Pts were evaluated for AEs/SAEs after DES. In addition, we calculated rates of transplantation, graft & patient survival for the DES and transplanted patients compared to 821 NS pts. Results: A total of 513 HS pts received desensitization with 1 or more doses of rituximab from 8/2005 to current. 396/513(77%) of pts received only 1 dose of rituximab pre-transplant, 91/513 (17.7%) received 2 doses, 22/513 (4.3%) received 3 doses and 4/513 patients received 4 or more doses (1 patient received 5 doses). Most importantly, 424/513 (82.6%) patients were transplanted. 89/513 (17.3%) patients not transplanted. 10/89 non-transplanted pts. expired. Adverse events occurred in 12/26 (46%) pts who received 3-5 doses of rituximab. Patient and graft survival for pts in the transplanted group are shown in figure 1.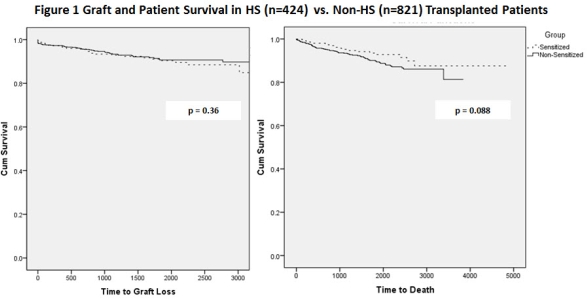 and compared to 821 NS patients transplanted during the same period. No significant differences were seen between the groups over this time period. Conclusion: Current UNOS rates of transplantation for HS patients w/o DES are <10%. Here we show that DES with IVIG + Rituximab can achieve rates of 82.6% over 10 years. In addition, patient and graft survival are similar to NS patients. Thus, DES offers the benefits of kidney transplantation to HS pts. who would otherwise have poorer survival on dialysis.
and compared to 821 NS patients transplanted during the same period. No significant differences were seen between the groups over this time period. Conclusion: Current UNOS rates of transplantation for HS patients w/o DES are <10%. Here we show that DES with IVIG + Rituximab can achieve rates of 82.6% over 10 years. In addition, patient and graft survival are similar to NS patients. Thus, DES offers the benefits of kidney transplantation to HS pts. who would otherwise have poorer survival on dialysis.
CITATION INFORMATION: Choi J, Kahwaji J, Vo A, Louie S, Kang A, Peng A, Villicana R, Puliyanda D, Kim I, Jordan S. Long-Term Assessment of the Safety and Efficacy of IVIG and Rituximab-Based Desensitization (DES). Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Choi J, Kahwaji J, Vo A, Louie S, Kang A, Peng A, Villicana R, Puliyanda D, Kim I, Jordan S. Long-Term Assessment of the Safety and Efficacy of IVIG and Rituximab-Based Desensitization (DES). [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/long-term-assessment-of-the-safety-and-efficacy-of-ivig-and-rituximab-based-desensitization-des/. Accessed February 15, 2026.« Back to 2016 American Transplant Congress
