Alemtuzumab Is Safe and Effective Induction Therapy in Highly Sensitized (HS) Pediatric Patients Undergoing Transplantation.
1Comprehensive Transplant Center, Cedars-Sinai Medical Center, Los Angeles, CA
2Biostatistics Core, Cedars-Sinai Medical Center, Los Angeles, CA.
Meeting: 2016 American Transplant Congress
Abstract number: D152
Keywords: Alloantigens, Highly-sensitized, Kidney transplantation, Pediatric
Session Information
Session Name: Poster Session D: Kidney-Pediatrics
Session Type: Poster Session
Date: Tuesday, June 14, 2016
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Halls C&D
Introduction: Studies show that alemtuzumab, a potent lymphocyte-depleting agent, is well-tolerated in pediatric renal transplantation. We report on the use of alemtuzumab induction in HS pediatric kidney transplant.
Methods: 50 renal transplants were performed from 1/2009-12/2014. 15 HS patients received IVIg/Rituximab for desensitization with alemtuzumab induction (15-30mg, 1 dose), while 35 nonsensitized patients received anti-IL-2R (2 doses). Graft survival and infections were compared between 2 groups.
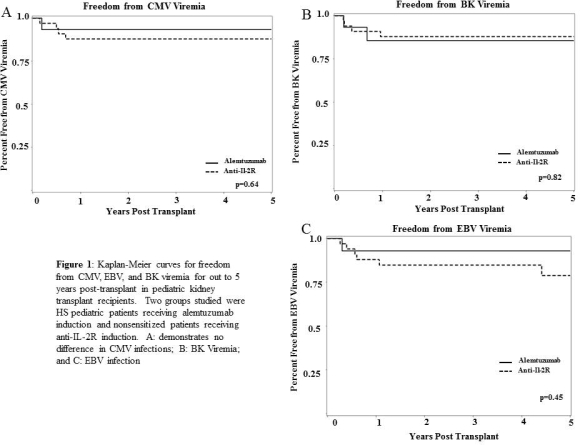
Results: All HS patients had received a prior transplant and were older. Patient survival was 100% and graft outcomes were similar with mean 1-year creatinine of 1.03±0.45 vs. 0.99±0.6, p=0.48. White blood cell (WBC) and absolute lymphocyte count (ALC) were significantly lower in alemtuzumab group at 30 days, p<0.0001, and at 1 year, p=0.026 and p=0.001, respectively. There was no significant difference in bacterial, viral (viremia defined as >50 copies/PCR), or fungal infections over 5 years.
Conclusions: Alemtuzumab induction with desensitization was well-tolerated and effective in HS pediatric patients. Although significant suppression of WBC and ALC up to 1 year post-transplant was observed, there was no significant increase in infections.
| Variable | Alemtuzumab (N=15) | Percent | IL-2R (N=35) | Percent | P-Value |
| Age, Median (IQR) | 18 (14 – 19) | 14 (5 – 17) | 0.012 | ||
| Sex | Female | 40 | Female | 51.4 | 0.54 |
| Living Donor | 1 | 6.7 | 3 | 8.6 | |
| Serum creatinine 12 months | 1.03±0.45 | 0.99±.60 | 0.48 | ||
| WBC | |||||
| Day 30 | 4.30±2.17 | 9.28±3.22 | < 0.0001 | ||
| Day 365 | 5.76±2.63 | 8.07±2.44 | 0.026 | ||
| ALC | |||||
| Day 30 | 0.15±0.16 | 3.94±2.26 | < 0.0001 | ||
| Day 365 | 1.49±2.12 | 2.97±1.24 | 0.001 | ||
| Infections | |||||
| Bacterial | 7 | 46.7 | 19 | 54.2 | NS |
| Fungal | 0 | 0 | |||
| Viral | 3 | 20 | 12 | 34.2 | NS |
CITATION INFORMATION: Kim I, Choi J, Vo A, Kang A, Toyoda M, Mirocha J, Kamil E, Louie S, Galera O, Jordan S, Puliyanda D. Alemtuzumab Is Safe and Effective Induction Therapy in Highly Sensitized (HS) Pediatric Patients Undergoing Transplantation. Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Kim I, Choi J, Vo A, Kang A, Toyoda M, Mirocha J, Kamil E, Louie S, Galera O, Jordan S, Puliyanda D. Alemtuzumab Is Safe and Effective Induction Therapy in Highly Sensitized (HS) Pediatric Patients Undergoing Transplantation. [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/alemtuzumab-is-safe-and-effective-induction-therapy-in-highly-sensitized-hs-pediatric-patients-undergoing-transplantation/. Accessed February 4, 2026.« Back to 2016 American Transplant Congress
