First Experience with Obinutuzumab (Type II Anti-CD20) in Patients with Treatment-Resistant Glomerular Diseases and Antibody-Mediated Rejection.
Comprehensive Transplant Center, Cedars-Sinai Medical Center, Los Angeles, CA.
Meeting: 2016 American Transplant Congress
Abstract number: D130
Keywords: B cells, CD20, Highly-sensitized, Nephrotic syndrome
Session Information
Session Name: Poster Session D: Kidney Immunosuppression: Novel Agents
Session Type: Poster Session
Date: Tuesday, June 14, 2016
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Halls C&D
Introduction: There is extensive experience with rituximab (RTX,Type I anti-CD20) for desensitization (DES) and treatment of ABMR. There is also a comparable experience in treatment of glomerulonephritis (GN) and nephrotic syndrome (NS). However, RTX may not always be effective. Here, we report on the first experience in GN and HLA sensitized patients (pts) with a novel glyco-engineered type II anti-CD20 (Obinutuzumab,OBI) that is more effective in depletion of B-cells than RTX. Methods: From 7/15, we identified 11 pts with primary GN & NS, recurrent GN & NS post-transplant and pts with RTX-resistant DES and ABMR who were subsequently treated with OBI. OBI was administered on day 1 (100mg), day 2 (900mg) and day 15 (1000mg) as per CLL dosing guidelines. Pre-medication with acetaminophen 650mg p.o., diphenhydramine 25mg p.o. and methylprednisolone 40 mg i.v were given. Pts were monitored for relevant clinical and laboratory parameters specific to their condition. These included: DSAs (pre & 1M post-treatment), auto-antibodies (anti-dsDNA & anti-AT1R) pre & 1M post-treatment, SCr, and degrees of proteinuria. Results: From a safety standpoint, OBI was very well tolerated in this non-B-cell malignancy population. No infusion AE/SAE were noted. One pt developed C. diff diarrhea 1M post-OBI that resolved. The details of responses to OBI treatment are shown in figure 1. 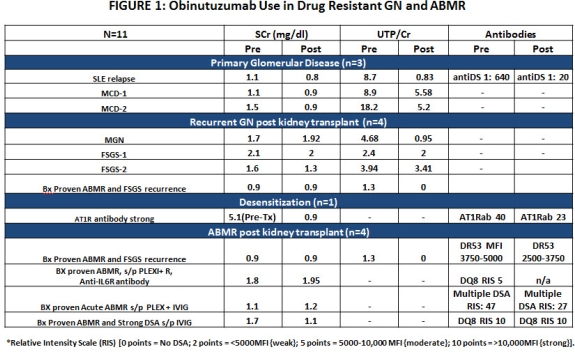 Briefly, 3 pts with primary GN & NS showed rapid and significant response to OBI. One pt with recurrent SLE showed rapid resolution of sxs & decline in anti-dsDNA antibodies (1:640 → 1:20) in 2 weeks. Pts with RTX-resistant MCD showed rapid improvements in proteinuria and resolution of NS in 2 cases. In 3 pts with recurrent GN, reduction in proteinuria and stabilization of SCr was seen. DSAs in 2/3 pts with RTX-resistant ABMR showed reductions in 2/3 pts with stable SCr. Conclusion: This early experience with OBI in renal transplant & GN pts suggests the drug appears safe and has efficacy beyond that seen with RTX. This is likely due to OBI's novel MOA that results in superior B-cell depletion compare to RTX.
Briefly, 3 pts with primary GN & NS showed rapid and significant response to OBI. One pt with recurrent SLE showed rapid resolution of sxs & decline in anti-dsDNA antibodies (1:640 → 1:20) in 2 weeks. Pts with RTX-resistant MCD showed rapid improvements in proteinuria and resolution of NS in 2 cases. In 3 pts with recurrent GN, reduction in proteinuria and stabilization of SCr was seen. DSAs in 2/3 pts with RTX-resistant ABMR showed reductions in 2/3 pts with stable SCr. Conclusion: This early experience with OBI in renal transplant & GN pts suggests the drug appears safe and has efficacy beyond that seen with RTX. This is likely due to OBI's novel MOA that results in superior B-cell depletion compare to RTX.
CITATION INFORMATION: Choi J, Jordan S, Vo A. First Experience with Obinutuzumab (Type II Anti-CD20) in Patients with Treatment-Resistant Glomerular Diseases and Antibody-Mediated Rejection. Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Choi J, Jordan S, Vo A. First Experience with Obinutuzumab (Type II Anti-CD20) in Patients with Treatment-Resistant Glomerular Diseases and Antibody-Mediated Rejection. [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/first-experience-with-obinutuzumab-type-ii-anti-cd20-in-patients-with-treatment-resistant-glomerular-diseases-and-antibody-mediated-rejection/. Accessed March 9, 2026.« Back to 2016 American Transplant Congress
