Long-Term Follow-Up of Renal Transplant Recipients (RTR) Treated with IVIG for De Novo Donor Specific Antibodies (DSA).
1Houston Methodist Hospital, Houston, TX
2Kansas University Medical Center, Kansas City, KS.
Meeting: 2016 American Transplant Congress
Abstract number: A231
Keywords: Alloantibodies, HLA antibodies, Immunoglobulins (Ig), Kidney transplantation
Session Information
Session Name: Poster Session A: Long Term Outcomes in Kidney Transplantation
Session Type: Poster Session
Date: Saturday, June 11, 2016
Session Time: 5:30pm-7:30pm
 Presentation Time: 5:30pm-7:30pm
Presentation Time: 5:30pm-7:30pm
Location: Halls C&D
Intravenous immune globulin (IVIG) may be beneficial for pre-emptive treatment of de novo DSA in RTR without biopsy-proven acute rejection (AR). Herein, we present long-term follow-up of stable RTR with DSA treated with IVIG. Methods: We prospectively monitored all RTR from 7/2007-7/2012 for DSA at 1, 3, 6, 9 and 12 months, then bi-annually. RTR positive for DSA on ≥2 occasions underwent biopsy. In 2012, recipients with DSA and no AR received IVIG 1 g/kg monthly x 6 months after attaining insurance approval (IVIG, n=12). Outcomes were compared to RTR denied insurance approval or who were otherwise unable to receive IVIG (No IVIG, n=14). Results: IVIG patients were more likely to be white, female, and living donor recipients (Table 1). Mean follow-up was 46±14 months post-DSA onset. One patient stopped IVIG after the 5th dose due to a flu-like reaction, otherwise IVIG was well-tolerated. DSA-related outcomes are shown below.
| Table 1. Baseline Characteristics | IVIG (n=12) | No IVIG (n=14) |
| Age, years | 50±13 | 48±13 |
|
Race, n(%) Black White |
4(33) 4(33) |
6(43) 2(14) |
| Female gender, n(%) | 8(67) | 6(43) |
| Living donor recipient, n(%) | 4(33) | 2(14) |
| Peak PRA, % | 47±41 | 35±35 |
| HLA mismatches/6 | 3.9±2 | 5±1 |
| Table 2. DSA-Related Outcomes | IVIG (n=12) | No IVIG (n=14) |
| BPAR following DSA, n(%) | 8 (67) | 5 (36) |
| Time DSA onset to BPAR, days | 500±453 | 596±349 |
| Graft loss, n(%) | 2(17) | 3(21) |
| Causes of graft loss |
Chronic TG (1) Death (1) |
IFTA following BPAR and chronic ureteral obstruction (1) Chronic rejection/diabetic nephropathy (1) Death (1) |
| Most recent Cr, mg/dL | 1.4±0.6 | 1.3±0.6 |
| DSA negative at last follow-up, n(%) | 4(33) | 7(50) |
| # of DSA specificities at last follow-up | 1.6±0.7 (n=8) | 1±0 (n=7) |
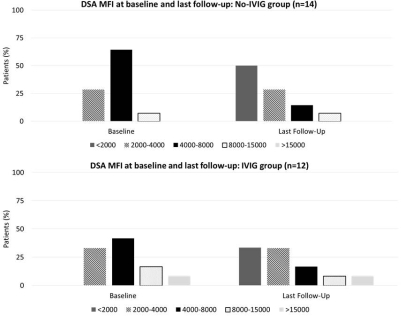 Conclusion: In a pilot study of IVIG for treatment of DSA, we did not observe any discernible impact on DSA clearance or MFI reduction. Graft function was similar to those who did not receive IVIG. Larger studies and/or alternate dosing regimens may be required to determine the role of IVIG for treatment of DSA.
Conclusion: In a pilot study of IVIG for treatment of DSA, we did not observe any discernible impact on DSA clearance or MFI reduction. Graft function was similar to those who did not receive IVIG. Larger studies and/or alternate dosing regimens may be required to determine the role of IVIG for treatment of DSA.
CITATION INFORMATION: Knight R, Patel S, Loucks-DeVos J, Kuten S, Gaber A. Long-Term Follow-Up of Renal Transplant Recipients (RTR) Treated with IVIG for De Novo Donor Specific Antibodies (DSA). Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Knight R, Patel S, Loucks-DeVos J, Kuten S, Gaber A. Long-Term Follow-Up of Renal Transplant Recipients (RTR) Treated with IVIG for De Novo Donor Specific Antibodies (DSA). [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/long-term-follow-up-of-renal-transplant-recipients-rtr-treated-with-ivig-for-de-novo-donor-specific-antibodies-dsa/. Accessed January 9, 2026.« Back to 2016 American Transplant Congress
