ASC-Deficient Dendritic Cells Exhibit Activation Defects and a Reduced Ability to Stimulate Allogeneic T Cells.
Medicine, University of California-San Diego, La Jolla, CA.
Meeting: 2016 American Transplant Congress
Abstract number: A23
Keywords: Ischemia, Proliferation, Rejection, T cells
Session Information
Session Type: Poster Session
Date: Saturday, June 11, 2016
Session Time: 5:30pm-7:30pm
 Presentation Time: 5:30pm-7:30pm
Presentation Time: 5:30pm-7:30pm
Location: Halls C&D
Donor dendritic cell (DC) activation causes adaptive immune responses that lead to rejection of transplanted allografts. Accumulating data suggest that inflammasome assembly within interstitial DCs in the donor organ is induced by ischemia-associated release of DAMPs/PAMPs. Following activation, DCs in the donor organ migrate to host LNs, activate T cells, and induce allograft rejection. Inflammasome assembly requires the adapter protein ASC for oligomerization. Upon assembly, the NLRP3 inflammasome induces the secretion of proinflammatory cytokines and the upregulation of DC chemokine/cytokine receptors. Our study sought to evaluate the role of inflammasome adaptor proteins in DC activation, migration and ability to activate naïve allogeneic T cells. Isolated DCs from WT vs. inflammasome deficient (NLRP3-/- and ASC-/-) mice (B6, H2b background) were stimulated with LPS for 5 days and examined for expression of CD80 and CD86 by FACS. LPS stimulated DCs were seeded in transwell chambers to test for migration in response to CCL21 (500ng/mL). A standard mixed lymphocyte response was used to assess the ability of WT vs. inflammasome-deficient DCs to stimulate allogeneic T cells (H2d).
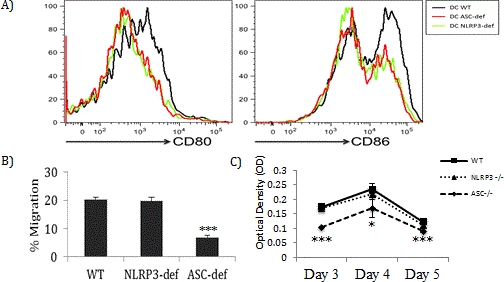
Upon LPS stimulation, CD80 and CD86 expression was reduced in ASC-/- and NLRP3-/- DCs compared to WT DCs (Figure 1A). ASC-/- DCs had significantly less chemokine-induced migration relative to WT and NLRP3-/- DCs (Figure 1B). We also noted a marked defect in ASC-/- T cell proliferation in response to alloantigens. Interestingly, DCs from WT and NLRP3-/- mice induced robust proliferation of allogeneic T cells whereas DCs from ASC-/- mice induced significantly reduced proliferation (Figure 1C).
Blockade of the inflammasome component ASC, but not NLRP3 resulted in significantly reduced DC migration, proliferation and ability to induce allogeneic T cell proliferation. Our data suggest that the adaptor protein ASC is a potential target to ameliorate ischemia-induced DC activation in donor allografts. We are currently seeking to further evaluate the role of the NLRP3 inflammasome and its component proteins in differing models of allograft rejection.
CITATION INFORMATION: Scheinok A, Shigeoka A, Kasimsetty S, Elahimehr R, McKay D. ASC-Deficient Dendritic Cells Exhibit Activation Defects and a Reduced Ability to Stimulate Allogeneic T Cells. Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Scheinok A, Shigeoka A, Kasimsetty S, Elahimehr R, McKay D. ASC-Deficient Dendritic Cells Exhibit Activation Defects and a Reduced Ability to Stimulate Allogeneic T Cells. [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/asc-deficient-dendritic-cells-exhibit-activation-defects-and-a-reduced-ability-to-stimulate-allogeneic-t-cells/. Accessed January 19, 2026.« Back to 2016 American Transplant Congress
