Variation in Immunosuppressive Regimen in Children Receiving Liver Transplants: An Analysis of the UNOS Database
1Pediatrics, University of Pittsburgh, Pittsburgh, PA, 2Starzl Network for Excellence in Pediatric Transplantation, Pittsburgh, PA, 3UPMC Children's Hospital of Pittsburgh, Pittsburgh, PA, 4Pediatrics, University of California San Francisco, San Francisco, CA
Meeting: 2022 American Transplant Congress
Abstract number: 329
Keywords: Immunosuppression, Liver transplantation, Survival
Topic: Clinical Science » Liver » 61 - Liver: Pediatrics
Session Information
Session Time: 5:30pm-7:00pm
 Presentation Time: 6:20pm-6:30pm
Presentation Time: 6:20pm-6:30pm
Location: Hynes Room 311
*Purpose: Optimizing immunosuppression (IS) was identified as a priority for pediatric liver transplant (LT) families and providers in the Starzl Network, a multi-center learning health system that aims to address pediatric LT challenges. We aimed to understand IS variation early post-LT and its impact on clinical outcomes.
*Methods: We conducted a retrospective analysis of United Network for Organ Sharing Standard Transplant Analysis and Research files in pediatric (0-18 years) liver-only transplant from 2013-2018. Analysis focused on early post-LT IS and 1 year post-LT outcomes. We compared (1) induction with steroids only vs non-T-cell depleting antibodies (NDA) vs T-cell depleting antibodies (DA) and (2) adjunct mycophenolate mofetil (MMF) or not. We examined (1) clinical and center predictors of IS and (2) IS impact on death (competing risks regression), graft loss (Cox proportional hazards), and rejection (logistic regression) up to 1 year post-LT.
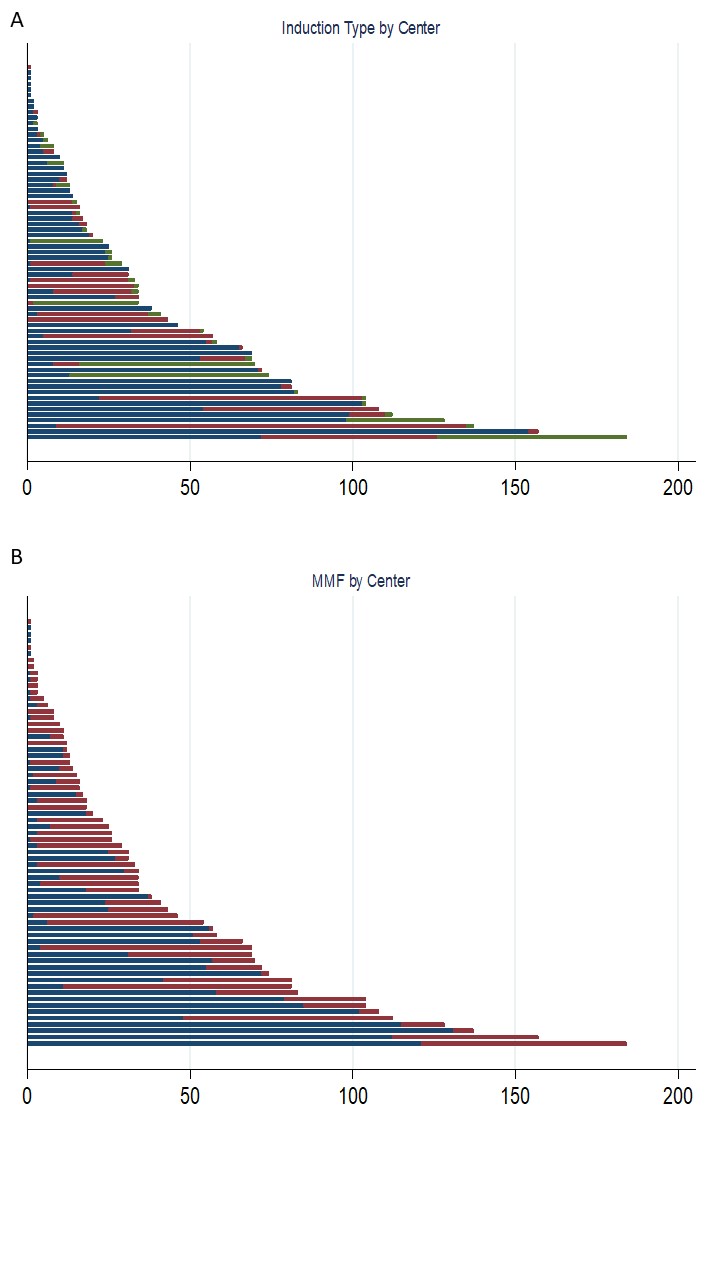
*Results: Among 2661 LT recipients, 1668 (62.7%) received steroids alone, 689 (25.9%) received NDA, and 304 (11.4%) received DA. MMF was used by 1050 (39.5%). Figures 1 shows variation in (A) induction and (B) MMF use by LT center. Steroid only induction was more common in LT for tumors (p=0.034), in medium volume centers (p<0.001), and prior to 2016 (p<0.001); DA was more common in cadaveric whole LTs (p<0.001). MMF use was more common in LT recipients age >11 years, non-biliary atresia cirrhosis, non-1B listing, low volume centers (p<0.001 for each), retransplant (p=0.002), or pre-LT intensive care (p=0.025). Compared to steroids only, unadjusted 1-year mortality was lower with DA (subdistribution hazard ratio [SHR] 0.36; 95% CI 0.15 - 0.89) but not NDA (SHR 0.98; 95% CI 0.64 - 1.50). One-year graft loss was lower with DA (hazard ratio [HR] 0.49; 95% CI 0.28 - 0.85) but not NDA (HR 0.99; 95% CI 0.74 - 1.33). MMF use was not associated with differences in 1-year mortality (SHR 0.72; 95% CI 0.48 - 1.08) or graft loss (HR 0.81; 95% CI 0.62 - 1.06). Neither induction type nor MMF use was associated with rejection by 1 year.
*Conclusions: Variation exists between centers in induction regimen and MMF use. Induction regimen may be associated with differential graft and patient survival but not rejection. Future work will look to isolate the effect of IS choice on key LT outcomes to inform quality patient care.
To cite this abstract in AMA style:
Raghu VK, Squires JE, Confair C, Katz-Eisenberg E, Mazariegos GV, Perito ER. Variation in Immunosuppressive Regimen in Children Receiving Liver Transplants: An Analysis of the UNOS Database [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/variation-in-immunosuppressive-regimen-in-children-receiving-liver-transplants-an-analysis-of-the-unos-database/. Accessed February 28, 2026.« Back to 2022 American Transplant Congress