Validation of a Prediction System for Risk of Allograft Loss (iBOX) in Pediatric Kidney Transplant Recipients
1Pediatric Nephrology, Robert Debré Hospital, APHP, Paris, France, 2Paris Transplant Group, INSERM, Paris, France, 3Pediatric Nephrology, Children Healthcare of Atlanta, Atlanta, GA, 4Pediatric Nephrology, Necker Hospital, Paris, France, 5UAB School of Medicine, Birmingham, AL, 6Pediatric Nephrology, Seattle Children, Seattle, WA, 7University Children's Hospital, Heidelberg, Germany, 8Medical University of South Carolina, Charleston, SC, 9Childrens Mercy Hospital, Kansas City, MO, 10Pediatric Nephrology, David Geffen School of Medicine at UCLA, Los Angeles, CA, 11Pediatric Nephrology, Le Bonheur Children's Hospital, Memphis, TN, 12Emory Transplant Center, Emory University, Atlanta, GA
Meeting: 2022 American Transplant Congress
Abstract number: 104
Keywords: Graft failure, Kidney transplantation, Pediatric, Prediction models
Topic: Clinical Science » Kidney » 43 - Kidney: Pediatrics
Session Information
Session Time: 5:30pm-7:00pm
 Presentation Time: 5:30pm-5:40pm
Presentation Time: 5:30pm-5:40pm
Location: Hynes Ballroom C
*Purpose: Kidney allograft loss is a common cause of end-stage renal disease but accurate prediction models of kidney allograft loss are lacking in children. The iBOX system has been broadly validated among adults. We aimed to validate the iBOX system in a large international cohort of pediatric kTx recipients.
*Methods: In this observational study, we used data from pediatric (<21) patients transplanted between 2005 and 2017 from 20 institutions in Europe and the United States. Patients with functional parameters (eGFR and UPCR), donor specific antibody and biopsy results (Banff scores g, ptc, cg, i, t, IFTA) were included. Individual predictions of allograft loss were obtained by applying the iBOX score on our data. The prediction performances of the model in our population were assessed via discrimination (c-statistics) and calibration.
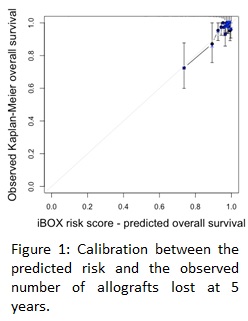
*Results: 573 kTx recipients were included. Median time from transplantation to evaluation was 1.0 [0.5-2.0] year with a mean age at evaluation at 12.1(5.5) years and mean follow-up after transplantation 5.1 (2.8) years. 5-year death-censored graft survival from evaluation was 95%. At the time of evaluation, mean eGFR and uPCR were 65.5(29.6)mL/min/1.73m2 and 0.25(1.2)g/g, respectively. 118 (20.6%) of the patients had DSA. The iBOX system showed good discrimination with a c-statistic of 0.81 and good calibration (Figure 1).
*Conclusions: The iBOX system demonstrated high accuracy in predicting kidney allograft loss in children with performances similar to those reported in adults.
To cite this abstract in AMA style:
Hogan J, Divard G, Garro R, Boyer O, Seifert M, Smith J, Toenshoff B, Twombley K, Warady B, Weng P, Zaar R, Patzer R, Loupy A. Validation of a Prediction System for Risk of Allograft Loss (iBOX) in Pediatric Kidney Transplant Recipients [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/validation-of-a-prediction-system-for-risk-of-allograft-loss-ibox-in-pediatric-kidney-transplant-recipients/. Accessed March 1, 2026.« Back to 2022 American Transplant Congress