Validating the Microarray Test for Antibody-Mediated Rejection in a Prospective Trial: The INTERCOM Study
University of Alberta, Edmonton, Canada
University of Minnesota, Minneapolis
Manchester Royal Infirmary, Manchester, United Kingdom
University of Maryland School of Medicine, Baltimore
Hospital de la Vall d'Hebron, Barcelona, Spain
Medical School of Hannover, Hannover, Germany
Meeting: 2013 American Transplant Congress
Abstract number: 29
We previously developed a microarray-based test for antibody-mediated rejection (ABMR) in biopsies from renal transplants. To validate it, we undertook the INTERCOM study, a prospective international multicenter study of central reading of 300 kidney transplant biopsies from six centers. The molecular measurements were expressed as the ABMR score assigned by the previously developed classifier equation. These were compared to the local diagnoses based on histology and donor-specific antibody (DSA), and with outcomes after biopsy. The results correlated with ABMR lesions in the biopsy: peritubular capillary inflammation (r=0.36, p=1.99e-10), glomerulitis (r=0.32, p=8.15e-09), and transplant glomerulopathy (TG) (r=0.47, p<2.2e-16), but did not tubulitis. The ABMR score was highest in biopsies from DSA positive patients (0.22 vs 0.10, p= 3.37e-05). Some errors in local readings emerged: 8 biopsies read as TG actually had ABMR (44%), and at least 6 ABMR were missed because no DSA was measured. The overall agreement with histology closely matched the pattern seen in the earlier reference set.
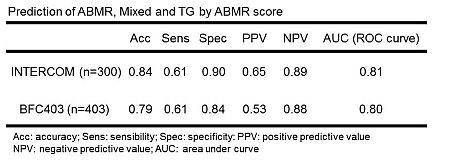
The ABMR score correlated with graft survival in Cox univariate analysis (coef=2.68, p=2.66e-15), and in multivariate analysis was more strongly associated with risk of failure (coef=2.72, p=4.35e-12) than was histology (coef=0.88, p=0.76) or DSA (coef=1.01, p=0.96). Some biopsies read as ABMR by histology-DSA had low ABMR scores, suggesting possible heterogeneity within ABMR: histologic lesions but no molecular activity. Centralized microarray-based biopsy assessment using predefined measurements can diagnose ABMR without knowledge of histology lesions and DSA, and predicts graft failure better than histology and DSA. The ABMR score circumvents the widespread disagreement on issues such as C4d testing by an objective and universal measurement.
To cite this abstract in AMA style:
Pereira A, Reeve J, Chang J, Matas A, Freitas Dde, Bromberg J, Sellares J, Einecke G, Halloran P. Validating the Microarray Test for Antibody-Mediated Rejection in a Prospective Trial: The INTERCOM Study [abstract]. Am J Transplant. 2013; 13 (suppl 5). https://atcmeetingabstracts.com/abstract/validating-the-microarray-test-for-antibody-mediated-rejection-in-a-prospective-trial-the-intercom-study/. Accessed July 1, 2025.« Back to 2013 American Transplant Congress
