Val-Ganciclovir Prophylaxis Delays Onset of EBV Viremia in the First Year Post-Solid Organ Transplantation in High-Risk Patients
S. Albatati,1 A. Sharma,2 S. Gantt,1 K. Haubrich,1 A. Wright,1 T. Blydt-Hansen.1
1University of British Columbia, Vancouver, Canada
2University of Manitoba, Winnipeg, Canada.
Meeting: 2018 American Transplant Congress
Abstract number: D156
Keywords: Epstein-Barr virus (EBV), High-risk, Prophylaxis, Viral therapy
Session Information
Session Name: Poster Session D: Kidney Infectious
Session Type: Poster Session
Date: Tuesday, June 5, 2018
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall 4EF
Introduction: Donor-derived primary Epstein-Barr virus (EBV) infection poses serious risks to EBV naïve children post-solid organ transplantation, including development of EBV-related PTLD. Currently, there is no consensus on the utility of antiviral prophylaxis. We examined the association between Val-ganciclovir prophylaxis (VAL) and onset of EBV viremia in the first six months after transplant, when immunosuppressant exposure is highest.
METHODS: Single center, retrospective study of EBV naïve pediatric heart and renal transplant recipients (n=44) with an EBV positive donor from January 1996- April 2017. Treatment exposure in patients who received VAL for CMV risk (n=28) was tested for association with viremia-free survival in the first 6 months post-transplantation. Survival models tested early exposure (minimum of 30 days) and duration of prophylaxis, with bivariate adjustment for other baseline confounders.
RESULTS: The cohort included 24 (55%) males, 2 (5%) heart transplants, aged 11.8 ± 6.25 years at transplant. 22 (50%) developed viremia in the first-year post-transplantation. Mean time-to-viremia (TTV) of 144 vs. 58 days for VAL, no-VAL respectively. VAL was associated with freedom from viremia (p=0.008) in the first 6 months 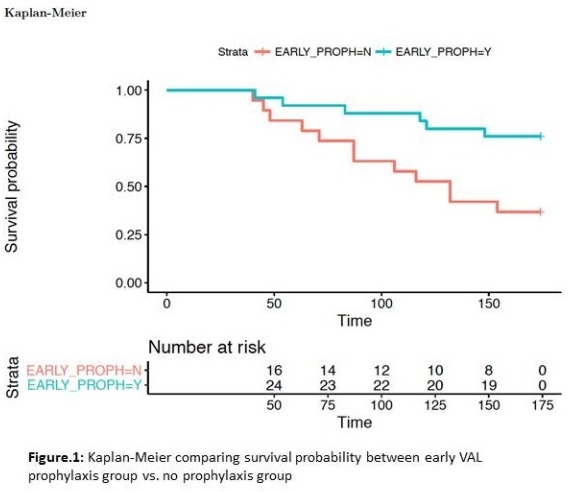 Only 3 out of 22 viremic patients developed viremia while on VAL, and those with delayed viremia had onset 39 ± 23 days after stopping VAL. Duration of VAL was associated with TTV (Cox, HR 0.986, 95% CI 0.977-0.994, p<0.001), with each additional day of VAL associated with 1.4% increase in viremia-free survival. Bivariate modelling of VAL with other baseline risk factors did not identify any other independent risk factors. Accelerated failure time modelling confirms the protective effect of VAL in the first year post-transplant, extending TTV by a factor of 1.0095 (p<0.001) for each day on treatment.
Only 3 out of 22 viremic patients developed viremia while on VAL, and those with delayed viremia had onset 39 ± 23 days after stopping VAL. Duration of VAL was associated with TTV (Cox, HR 0.986, 95% CI 0.977-0.994, p<0.001), with each additional day of VAL associated with 1.4% increase in viremia-free survival. Bivariate modelling of VAL with other baseline risk factors did not identify any other independent risk factors. Accelerated failure time modelling confirms the protective effect of VAL in the first year post-transplant, extending TTV by a factor of 1.0095 (p<0.001) for each day on treatment.
CONCULSION: VAL prophylaxis is associated with delayed onset of EBV viremia, with incremental benefit from each additional day of treatment.
CITATION INFORMATION: Albatati S., Sharma A., Gantt S., Haubrich K., Wright A., Blydt-Hansen T. Val-Ganciclovir Prophylaxis Delays Onset of EBV Viremia in the First Year Post-Solid Organ Transplantation in High-Risk Patients Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Albatati S, Sharma A, Gantt S, Haubrich K, Wright A, Blydt-Hansen T. Val-Ganciclovir Prophylaxis Delays Onset of EBV Viremia in the First Year Post-Solid Organ Transplantation in High-Risk Patients [abstract]. https://atcmeetingabstracts.com/abstract/val-ganciclovir-prophylaxis-delays-onset-of-ebv-viremia-in-the-first-year-post-solid-organ-transplantation-in-high-risk-patients/. Accessed February 20, 2026.« Back to 2018 American Transplant Congress
