Utilization of Organs from Donors Exposed to Hepatitis C: ? Standard of Care
M. Marvin1, S. Gaines2, P. Mohan3, A. Kotru1, C. Koo1, B. Addissie4, A. Unzueta4, S. Khurana4
1Transplantation and Liver Surgery, Geisinger, Danville, PA, 2Pharmacy, Geisinger, Danville, PA, 3Nephrology, Geisinger, Danville, PA, 4Hepatology, Geisinger, Danville, PA
Meeting: 2020 American Transplant Congress
Abstract number: B-198
Keywords: Donors, marginal, Hepatitis C, Kidney/liver transplantation, Viral therapy
Session Information
Session Name: Poster Session B: Non-Organ Specific: Viral Hepatitis
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
*Purpose: To report the results of a clinical program to utilize livers and kidneys from donors exposed to Hepatitis C.
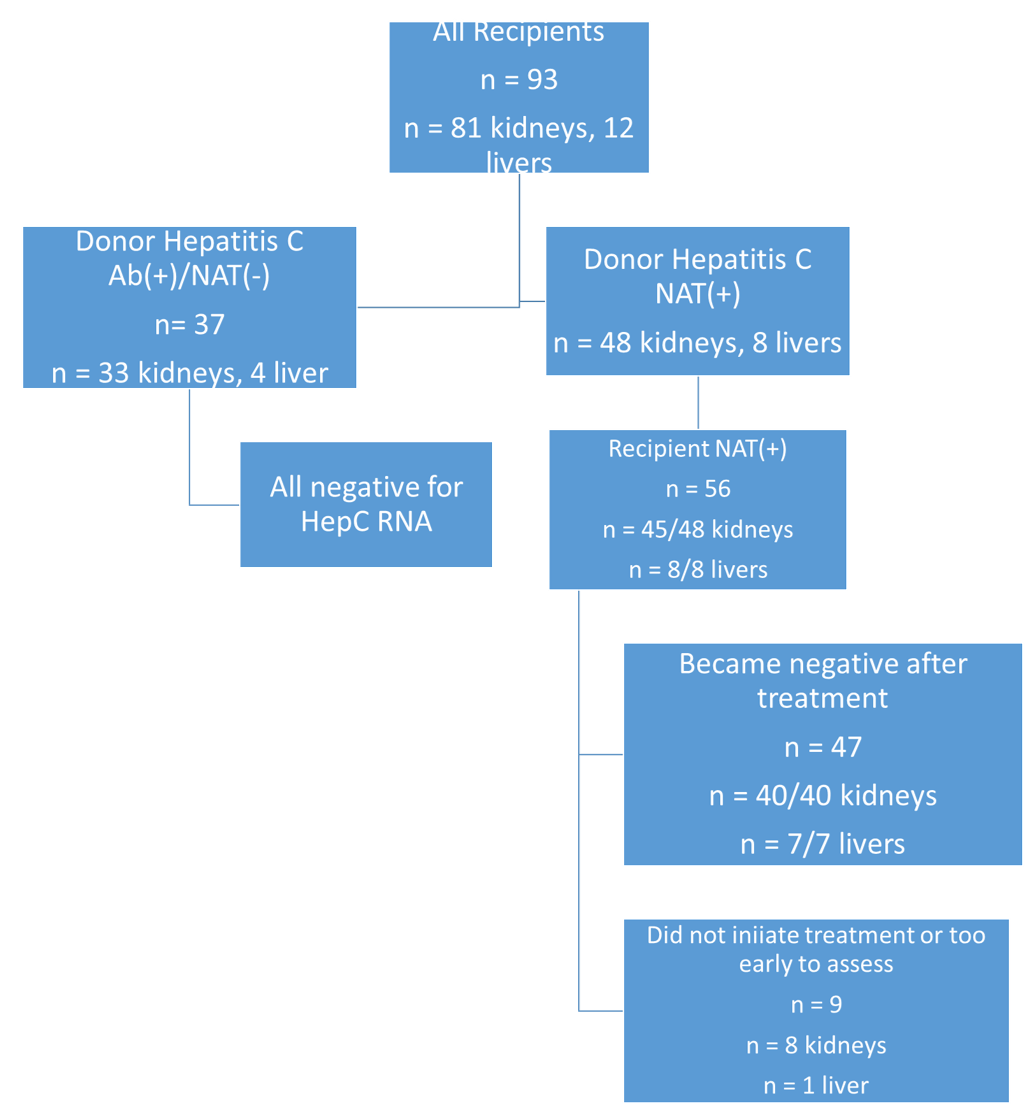
*Methods: We initially started to explore the safety of donor organs exposed to hepatitis C by only accepting antibody positive (Ab+)/Nucleic Acid Testing negative (NAT-) organs in July, 2017. In October, 2018, based on our initial experience and the data from clinical trials, and after gaining approval from Geisinger’s insurance company to cover the direct acting antiviral glecaprevir/pibrentasvir,, we began accepting NAT(+) organs for all consented candidates as part of our clinical practice. There was no preoperative assessment of donor hepatitis C genotype. When available, donor samples were tested for HCV RNA levels and genotype. All recipients were tested for HCV RNA and genotype within the first week after transplant. All patients received glecaprevir/pibrentasvir treatment as soon as the medications were available. All patients signed informed consent prior to being offered the organ.
*Results: A total of 93 patients were transplanted with 12 livers and 81 kidneys; 37 antibody positive/NAT(-) (4 liver, 33 kidney), 56 NAT(+) (8 livers, 48 kidneys). No recipient of a HepC Ab(+)/NAT(-) donor developed HCV RNA.
For those receiving a NAT(+) organ, mean (median) days to initiation of antiviral treatment was 13.3 (9.5) days. Hepatitis C genotypes included 1a, 1b, 2a, 2b, 3, 4, and unknown. All patients treated for at least 60 days cleared the virus. Mean (median) time from initiation of therapy to clearance of the virus was 38.8 (34.0) days for kidney recipients and 32.1 (29.0) days for liver recipients.
No patients required antiviral treatment cessation due to complications of the medications. No graft was lost or patient death attributed to hepatitis C treatment or disease.
*Conclusions: With the availability of direct acting agents, utilization of kidneys and livers from donors exposed to hepatitis C is safe and may be considered standard of care.
To cite this abstract in AMA style:
Marvin M, Gaines S, Mohan P, Kotru A, Koo C, Addissie B, Unzueta A, Khurana S. Utilization of Organs from Donors Exposed to Hepatitis C: ? Standard of Care [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/utilization-of-organs-from-donors-exposed-to-hepatitis-c-standard-of-care/. Accessed February 19, 2026.« Back to 2020 American Transplant Congress