Utility of Donor-Derived Cell Free DNA for Detecting ABMR in Patients With AT1R Antibodies
1Johns Hopkins University School of Medicine, Baltimore, MD, 2VCU, Richmond, VA, 3Wash Univ, St. Louis, MO, 4CareDx, Brisbane, CA
Meeting: 2021 American Transplant Congress
Abstract number: 1031
Keywords: Antibodies, Rejection
Topic: Clinical Science » Kidney » Kidney Acute Antibody Mediated Rejection
Session Information
Session Name: Kidney Acute Antibody Mediated Rejection
Session Type: Poster Abstract
Session Date & Time: None. Available on demand.
Location: Virtual
*Purpose: The presence of angiotensin II type 1 receptor (AT1R) antibodies is associated with non-HLA active antibody mediated rejection (ABMR). The frequent co-existence of negative donor-specific antibodies (DSAs) and histological features of ABMR with positive AT1R antibodies underlies the need for non-invasive markers of rejection. We investigated the utility of donor derived cell free DNA (dd-cfDNA) in patients with positive AT1R antibodies and ABMR.
*Methods: We performed a multicenter retrospective analysis of patients with positive AT1R antibodies who had concomitant dd-cfDNA measurements (AlloSure, CareDx) for surveillance or worsening of allograft function concerning for rejection. These patients also underwent allograft biopsies demonstrating evidence of ABMR with negative DSAs. A positive AT1R test was defined as >10 IU/mL and dd-cfDNA >1%. Statistical analysis included Spearman correlation and Mann-Whitney tests
*Results: We identified 16 kidney transplant recipients with histologic evidence of ABMR with negative DSA and positive AT1R antibodies- 6 (38%) were female, 7 (44%) were Caucasian (44%) and 7 (44%) were African American, with a mean age 43 years at transplant. Thirteen patients (81%) underwent deceased donor kidney transplantation. Primary FSGS was the etiology of kidney disease in the majority of patients.
All patients had elevation of dd-cfDNA >1% prior to allograft biopsy with median levels 2.6% (0.66-7.9%). There was an inverse association between levels of AT1R and dd-cfDNA (r=-0.2, p=0.2),with stronger correlation for dd-cfDNA done for concern for rejection (r=- 0.5, p= 0.12) compared to those done for all purposes. Levels of dd-cfDNA were lower in patients with FSGS as primary disease (p=0.04), in comparison other etiologies of kidney disease.
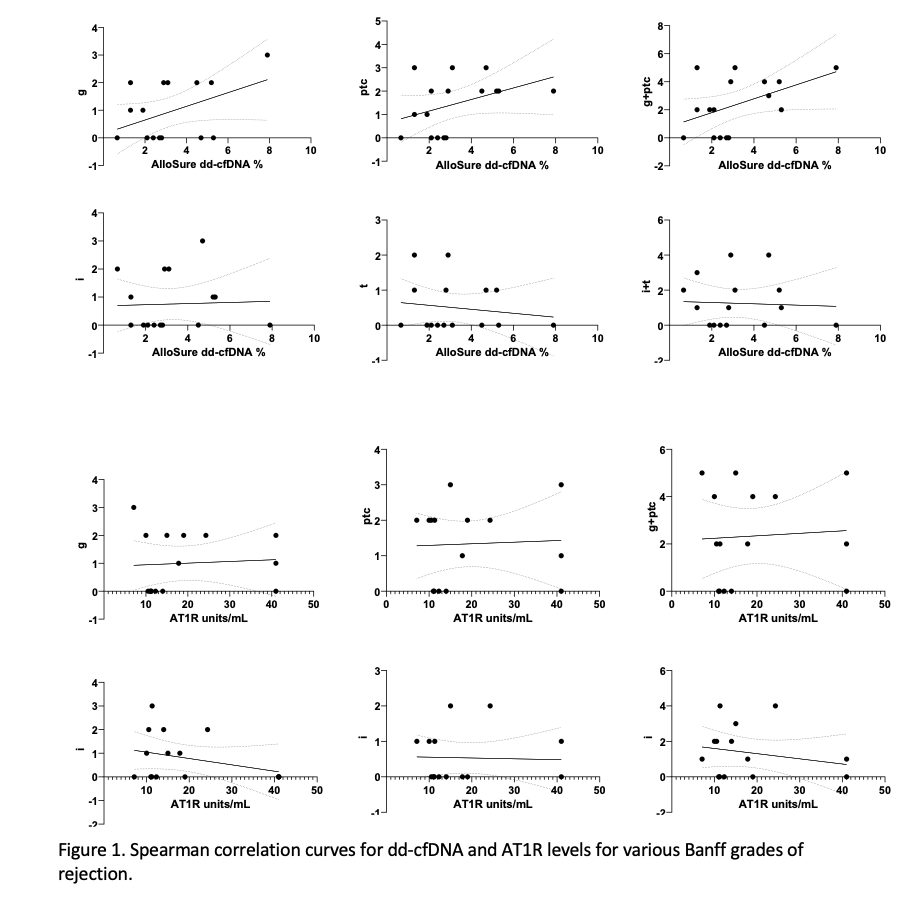
Dd-cfDNA levels correlated well with Banff grades of rejection (g r=0.3 p=0.12; ptc r=0.4 p=0.05; g+ptc r=0.4 p=0.04, i+t r=0.06, p=0.4), with AT1R levels demonstrating no correlation (Fig 1)
*Conclusions: This study suggests that AT1R titers do not reflect severity of ABMR, while dd-cfDNA correlates well with severity grades of the Banff criteria. This study demonstrates that dd-cfDNA could be used for monitoring and detecting rejection in patients with AT1R antibodies.It is imperative to conduct larger studies to validate these findings.
To cite this abstract in AMA style:
Kant S, Kumar D, Moinuddin I, Alhamad T, Haris M, Gray J, Dale B, Bettinotti M, Brennan DC. Utility of Donor-Derived Cell Free DNA for Detecting ABMR in Patients With AT1R Antibodies [abstract]. Am J Transplant. 2021; 21 (suppl 3). https://atcmeetingabstracts.com/abstract/utility-of-donor-derived-cell-free-dna-for-detecting-abmr-in-patients-with-at1r-antibodies/. Accessed February 27, 2026.« Back to 2021 American Transplant Congress