Use of De Novo mTOR Inhibitors in Highly Sensitized Kidney Transplant Recipients
1Tranplant Unit, Santa Casa De Misericordia De Juiz de Fora, Juiz de Fora, Brazil, 2Transplant Unit, Santa Casa De Misericórdia De Juiz de Fora, Juiz de Fora, Brazil, 3Tranplant Unit, Santa Casa De Misericórdia De Juiz de Fora, Juiz de Fora, Brazil
Meeting: 2022 American Transplant Congress
Abstract number: 1702
Keywords: Antibodies, Graft survival, Kidney transplantation, Rejection
Topic: Clinical Science » Kidney » 38 - Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Information
Session Name: Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Type: Poster Abstract
Date: Tuesday, June 7, 2022
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
*Purpose: mTOR inhibitors (mTORi) allow calcineurin-inhibitor reduction without loss of efficacy and may improve allograft-transplant outcomes. However, the safety of this protocol in high-immunological risk kidney transplants is not clear.
*Methods: To shed light on this issue, we examined 106 consecutive kidney transplant only with a baseline calculated panel reactive antibody (PRA) >= 20% or Donor Specific Antibody (DSA) prior to the transplant from Jan 2016 to December 2020 in our unit. Immunosuppression was based on CNI (tacrolimus), steroids and alternatively mycophenolic acid (MPA; n = 20), or mTORi (either everolimus or sirolimus, n = 96, target trough levels 4-8 ng/mL). Patients received methylprednisonlone and thymoglobulin induction. Outcome measures included a one-year analysis of patient and renal allograft survival and acute rejection incidence (biopsy-proven acute rejection – BPAR).
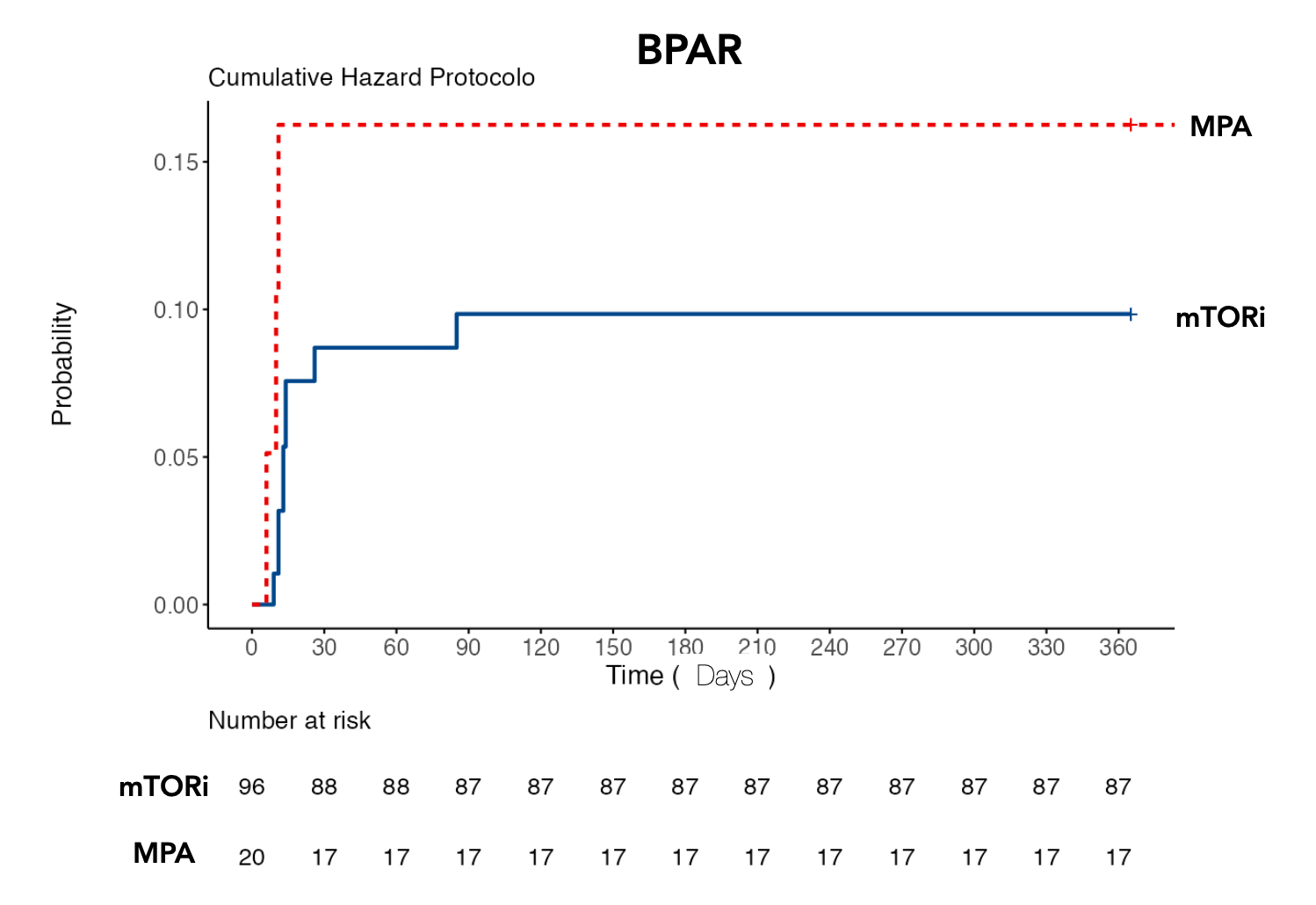
*Results: Demographic and immunological risk profiles were similar in both groups. Cumulative patient and graft survival were not significantly different in the two groups (mTORi: 82.5% and MPA 84% log-rank test p = 0.867). Cumulative incidence of BPAR was not significantly different ( mTORi: 10% and MPA: 17%; log-rank test p = 0.4).
*Conclusions: This single-center retrospective cohort one-year analysis suggests that in hypersensitized kidney transplant recipients receiving tacrolimus-based immunosuppressive therapy, similar clinical outcomes may be obtained using mTOR inhibitors compared to mycophenolate.
To cite this abstract in AMA style:
Bastos J, Freesz T, Bisi C, Marinho C, Pires A, Colares V, Neto R, Ferreira G. Use of De Novo mTOR Inhibitors in Highly Sensitized Kidney Transplant Recipients [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/use-of-de-novo-mtor-inhibitors-in-highly-sensitized-kidney-transplant-recipients/. Accessed March 4, 2026.« Back to 2022 American Transplant Congress