Use of Dd-cfdna to Guide Tapering of Immunosuppression Therapy in Kidney Transplant Recipients
1Victoria Kidney and Dialysis Associates, Victoria, TX, 2Natera, Inc., Austin, TX
Meeting: 2022 American Transplant Congress
Abstract number: 1683
Keywords: Dosage, Immunosuppression, Kidney transplantation, Mycophenolate mofetil
Topic: Clinical Science » Kidney » 38 - Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Information
Session Name: Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Type: Poster Abstract
Date: Tuesday, June 7, 2022
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
*Purpose: Continuous administration of immunosuppressive therapy (IST) is required to avoid allograft rejection in transplant recipients. However, these therapeutic interventions can lead to significant side effects including infections and cancers. Lowering IST can promote favorable outcomes following transplantation, but comes with an attendant risk of rejection. Donor derived cell free DNA (dd-cfDNA) is a validated non-invasive biomarker for active renal allograft rejection. In this observational study we analyzed whether dd-cfDNA monitoring was helpful to an independent physician in guiding IST reduction and minimizing risk for allograft rejection.
*Methods: Adult kidney transplant (KT) patients between 1 and 5 years post-KT, who were considered low-risk for rejection by the physician were enrolled and followed-up monthly for 7 visits. At each visit, IST treatment and dosages, serum creatinine, eGFR were recorded and dd-cfDNA levels were measured using the ProsperaTM test (Natera, Inc.). IST was lowered or modified per physician discretions. Allograft outcomes including adverse events were recorded at the last visit (visit 7).
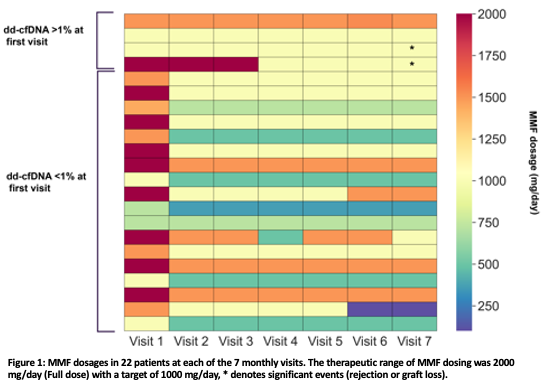
*Results: 24 KT patients were enrolled in this study. At visit 1, dd-cfDNA fractions were <1% for 19 patients and were elevated ≥1% for 5 patients. Throughout the entire study, dd-cfDNA fractions remained <1% for all patients with dd-cfDNA <1% initially, and remained ≥1% for 3/5 patients with initially elevated dd-cfDNA fractions. At the start of the study, ISTs administered by independent physicians were MMF + tacrolimus (n=20), MMF + tacrolimus + steroids (n=2), cyclosporine + steroids (n=1) and steroids + tacrolimus (n=1). Reduction in MMF dosing was the most common IST modification, with a target dose of 1000 mg/day (Figure 1). Among the 22 patients administered MMF (dd-cfDNA <1%: n=18, dd-cfDNA ≥1%: n=4), dosage was adjusted for 17 patients with dd-cfDNA <1%, and 1 patient with dd-cfDNA ≥1%. Dosage of MMF remained constant in 3/4 of the patients with dd-cfDNA ≥1% (Figure 1). Among the patients for which MMF was administered, adverse events (rejection and/or graft failure) occurred in 50% (2/4) of patients with dd-cfDNA ≥1% at the start of the study and in 0% (0/18) of patients with dd-cfDNA <1% (Figure 1*).
*Conclusions: dd-cfDNA measurement throughout the study identified individuals who were at higher risk for adverse events. This observational study shows that clinicians can use dd-cfDNA surveillance to inform their own decision making in adjusting IST while minimizing allograft rejection.
To cite this abstract in AMA style:
Osuchukwu G, Trevino A, Beretich L, McCormick S, Kaur N, White R, Gauthier P. Use of Dd-cfdna to Guide Tapering of Immunosuppression Therapy in Kidney Transplant Recipients [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/use-of-dd-cfdna-to-guide-tapering-of-immunosuppression-therapy-in-kidney-transplant-recipients/. Accessed March 7, 2026.« Back to 2022 American Transplant Congress