Urine Cell-Free Supernatant Metabolites Diagnostic of Antibody Mediated Rejection in Kidney Allografts.
1Weill Cornell Medicine, NY
2Weill Cornell Medical College-Qatar, Doha, Qatar.
Meeting: 2016 American Transplant Congress
Abstract number: 124
Keywords: Antibodies, Kidney transplantation, Rejection
Session Information
Session Name: Concurrent Session: Kidney AMR: Making the Diagnosis
Session Type: Concurrent Session
Date: Sunday, June 12, 2016
Session Time: 4:30pm-6:00pm
 Presentation Time: 5:42pm-5:54pm
Presentation Time: 5:42pm-5:54pm
Location: Veterans Auditorium
Introduction:
Urine cell-free supernatant metabolite profiles provide a unique opportunity to interrogate intra-graft processes and have distinguished acute cellular rejection from no rejection in the Clinical Trials in Organ Transplantation-04 study. Antibody mediated rejection (AMR) has emerged as a major cause of kidney allograft failure; however, the critical issue of urine metabolite profiles as diagnostic of AMR in kidney transplantation has not been investigated.
Methods:
We performed untargeted metabolite profiling of 141 urine cell-free supernatants matched to biopsy-proven AMR (N=20 specimens from 20 patients), acute tubular injury (ATI) (N=61 specimens from 56 patients), or normal biopsy result (NORMAL) (N=60 specimens from 29 patients). We evaluated whether the previously discovered diagnostic biomarkers of ACR (3-sialyllactose [3SL], xanthosine [X], quinolinate [QUIN], and X-16397, 3SL/X, and QUIN/X16397) distinguish: (i) patients with AMR biopsies from the patients with ATI biopsies and (ii) patient with AMR biopsies from patients with normal biopsies.
Results:
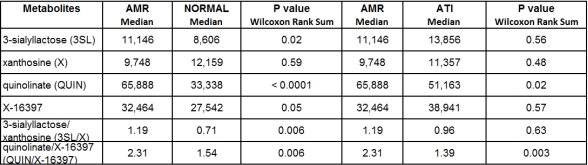
Osmolarity-corrected 3SL, QUIN, and X-16397 were significantly higher in the AMR Group than in the NORMAL Group (see Table 1; P values: 0.02, <0.0001, and 0.05, respectively). Osmolarity-corrected QUIN was also significantly higher in the AMR Group than in the ATI Group (P value 0.02). The ratio of QUIN/X-16397 distinguished the AMR group from the Normal Group (median 2.31 vs. 1.54, P value 0.006) and AMR group from the ATI Group (median 2.31 vs. 1.39, P value 0.003) (Table 1).
Conclusion:
With the use of a comprehensive combination of nontargeted LC-MS/MS and GC-MS based metabolomics platforms, we demonstrate that the relative abundance of quinolinate and the ratio of quinolinate to X-16397 distinguish patients with AMR biopsies from patients with Normal biopsies and patients with AMR from patients with ATI biopsies. Quinolinate, a product of tryphtophan metabolism, by serving as a precursor for the biogenesis of NAD+, may help meet the metabolic demands of immune cells involved in allograft rejection.
CITATION INFORMATION: Alkadi M, Lee J, Dadhania D, Muthukumar T, Snopkowski C, Li C, Salvatore S, Seshan S, Suhre K, Suthanthiran M. Urine Cell-Free Supernatant Metabolites Diagnostic of Antibody Mediated Rejection in Kidney Allografts. Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Alkadi M, Lee J, Dadhania D, Muthukumar T, Snopkowski C, Li C, Salvatore S, Seshan S, Suhre K, Suthanthiran M. Urine Cell-Free Supernatant Metabolites Diagnostic of Antibody Mediated Rejection in Kidney Allografts. [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/urine-cell-free-supernatant-metabolites-diagnostic-of-antibody-mediated-rejection-in-kidney-allografts/. Accessed July 12, 2025.« Back to 2016 American Transplant Congress
