Unclear Implications of Tacrolimus Time in Therapeutic Range as a Predictor of Acute Rejection in Renal Transplant Recipients Undergoing Early Corticosteroid Withdrawal
University of Illinois at Chicago, Chicago, IL
Meeting: 2021 American Transplant Congress
Abstract number: 818
Keywords: Calcineurin, Kidney/pancreas transplantation
Topic: Clinical Science » Pharmacy » Non-Organ Specific: Pharmacogenomics / Pharmacokinetics
Session Information
Session Name: Non-Organ Specific: Pharmacogenomics / Pharmacokinetics
Session Type: Poster Abstract
Session Date & Time: None. Available on demand.
Location: Virtual
*Purpose: Tacrolimus (TAC) demonstrates a wide intra- and inter-patient variability (IPV) necessitating therapeutic drug monitoring. The purpose of this study is to determine the impact of tacrolimus time in therapeutic range (TTR) in renal transplant (RT) recipients undergoing early corticosteroid withdrawal (ECSWD).
*Methods: Adult RT recipients at University of Illinois Hospital & Health Sciences System between 1/1/2015 – 12/31/2018 were included. Patients were excluded if they underwent ABO incompatible RT, positive flow crossmatch RT, died <90 days post-RT, were lost to follow-up <12 months post-RT, and deviated from standard institution protocol. TAC TTR was calculated using the Rosendaal method with protocol goal TAC levels (erroneous levels were excluded). High TTR (TTR-H) was defined as >75% based on the median of the cohort. Primary outcome was to compare the incidence of acute rejection (AR) between TTR-H and low TTR (TTR-L) 12 months post-RT. Secondary outcomes included comparing the incidence of donor-specific antibodies (DSA) and de novo DSA (dnDSA) and eGFR. Multivariate analyses were conducted to assess risk factors for dnDSA formation and AR.
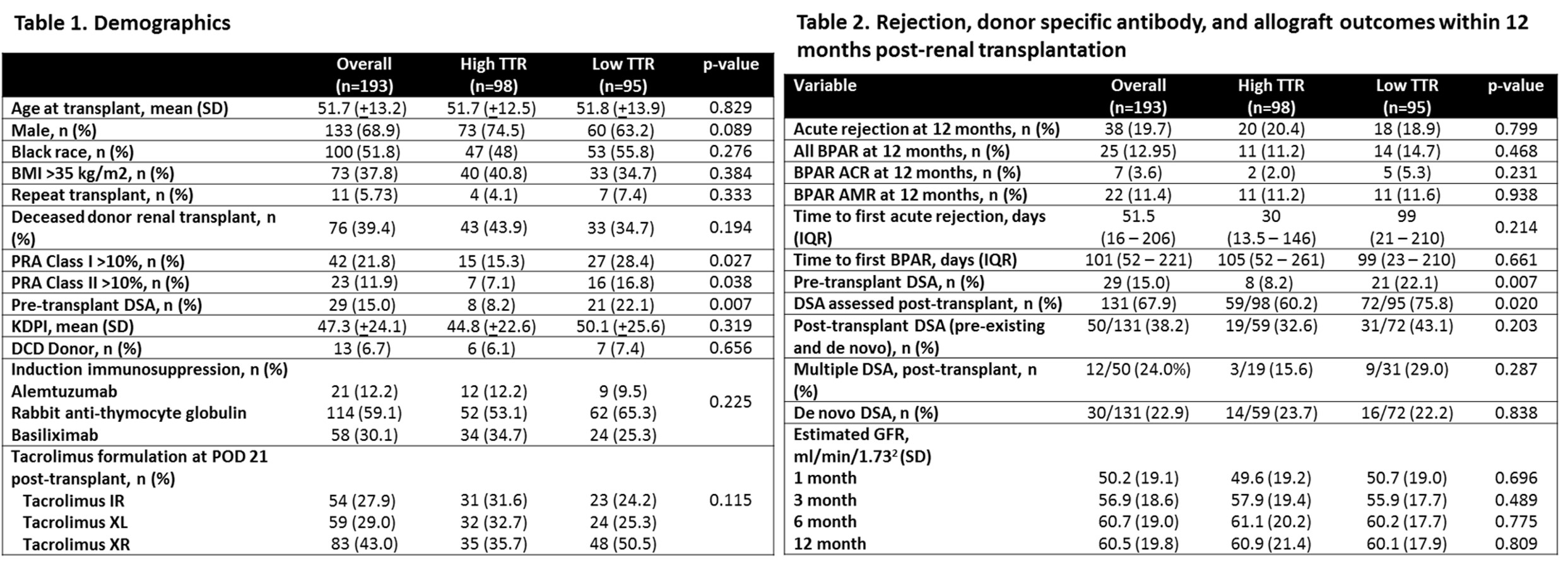
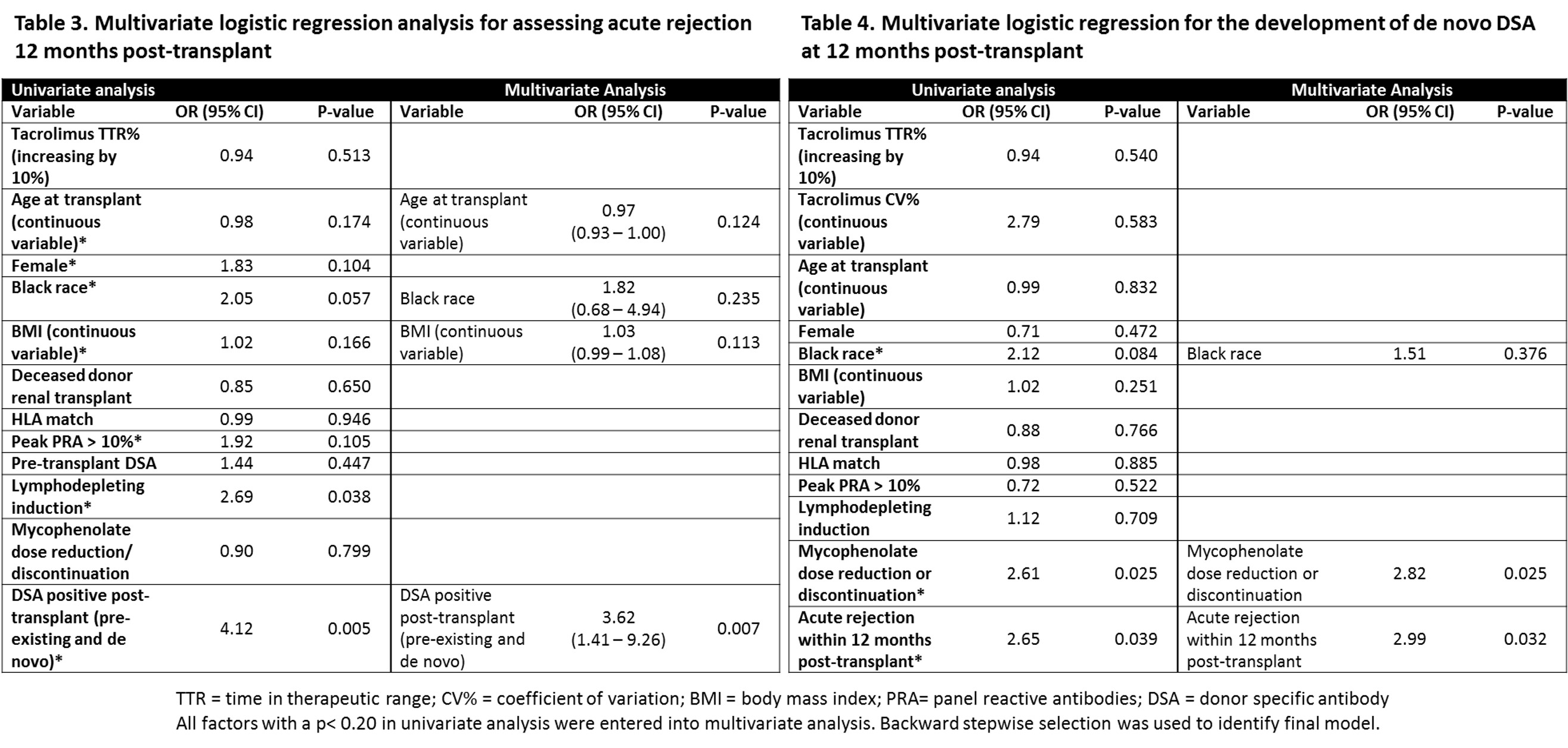
*Results: Demographics are represented in Table 1. Acute rejection rates were similar between groups (TTR-H 20.4% vs TTR-L 18.9%, p=0.799). Rejection subtype, DSA, and eGFR comparisons are detailed in Table 2. Positive DSA post-transplant (OR 3.62 95% CI 1.41 – 9.26, p=0.007) was associated with a higher AR at 12 months post-RT (Table 3). Mycophenolate dose reduction/discontinuation (OR 2.82 CI 95% 1.13 – 6.97, p=0.025) and AR (OR 2.99, 95% CI 1.09 – 8.18, p=0.032) were associated with increased dnDSA formation within 12 months of RT. Tacrolimus TTR did impact AR or dnDSA formation in univariate or multivariate modeling.
*Conclusions: In an ECSWD population, there was no difference in AR 12-months post-RT between TTR-H and TTR-L. There was a higher incidence of DSA at 1-year post-transplant in those with reduced mycophenolate dosing and history of acute rejection. Ideal population utility of tacrolimus TTR remains to be defined in RT with future studies.
To cite this abstract in AMA style:
Pierce D, West-Thielke P, Hajjiri Z, Gaitonde S, Tzvetanov I, Benedetti E, Lichvar A. Unclear Implications of Tacrolimus Time in Therapeutic Range as a Predictor of Acute Rejection in Renal Transplant Recipients Undergoing Early Corticosteroid Withdrawal [abstract]. Am J Transplant. 2021; 21 (suppl 3). https://atcmeetingabstracts.com/abstract/unclear-implications-of-tacrolimus-time-in-therapeutic-range-as-a-predictor-of-acute-rejection-in-renal-transplant-recipients-undergoing-early-corticosteroid-withdrawal/. Accessed February 18, 2026.« Back to 2021 American Transplant Congress