Two Similar Risk Models Identify Different Patients for Graft Loss: Implications for Clinical Trials in DSA+ Kidney Transplant Recipients
William J von Liebig Transplant Center, Mayo Clinic, Rochester.
Meeting: 2018 American Transplant Congress
Abstract number: C18
Keywords: Antibodies, Kidney transplantation, Rejection, Sensitization
Session Information
Session Name: Poster Session C: Kidney Chronic Antibody Mediated Rejection
Session Type: Poster Session
Date: Monday, June 4, 2018
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall 4EF
Introduction. Kidney Transplant Recipients (KTx) with donor-specific alloantibody (DSA) at transplantation have increased death-censored graft failure (DCGF) due to chronic antibody mediated rejection (cABMR), but graft losses due to other causes may complicate clinical trial design and results. The goal of the current study was to develop a prediction model of DCGF in DSA+ KTx to facilitate studies of cABMR.
Methods. KTx who had DSA at the time of surgery (DSA MFI >3000 and a negative T cell AHG crossmatch) with a functioning graft at 1 year were assessed for factors associated with DCGF between 1-5 years using 1 year clinical, laboratory and histologic data (surveillance biopsy). The discovery set included 147KTx at Mayo Clinic Rochester, (2000-2010) with 20.4% (30/147) who had DCGF. The validation set included 111KTx at Necker Hôpital, France (2003-2011) with 31.5% (33/111) who had DCGF.
Results. An existing model developed in low-risk recipients (Moreno et al, JASN 2016) gives C-statistic = 0.78 for DCGF in the Mayo discovery set and 0.86 in the Paris validation set, but does not differentiate for cause of failure. Two risk models with similar prediction of failure were identified using one year protocol data using 1) ACR and eGFR (C-stat 0.77) and 2) transplant glomerulopathy and interstitial fibrosis (C-stat 0.75).Combined together, these models excluded patients without allograft failure (n=82, 55.8%, Fig 1A). The histology model better predicted DCGF secondary to cABMR (Fig1B), whilst ACR and eGFR alone was associated with recurrent disease as cause of failure.
Conclusions. Two models with similar predictability can be constructed in DSA+ recipients, adapting from previous models in order to help identify etiology of graft failure. Using histology alone, patients with graft loss due to cABMR were predicted. Combining these models correctly identified 55.8% patients with no graft failure. This approach enriches a potential study population and thus might be preferable when considering entry criteria into clinical trials aimed to improve antibody-related DCGF outcomes.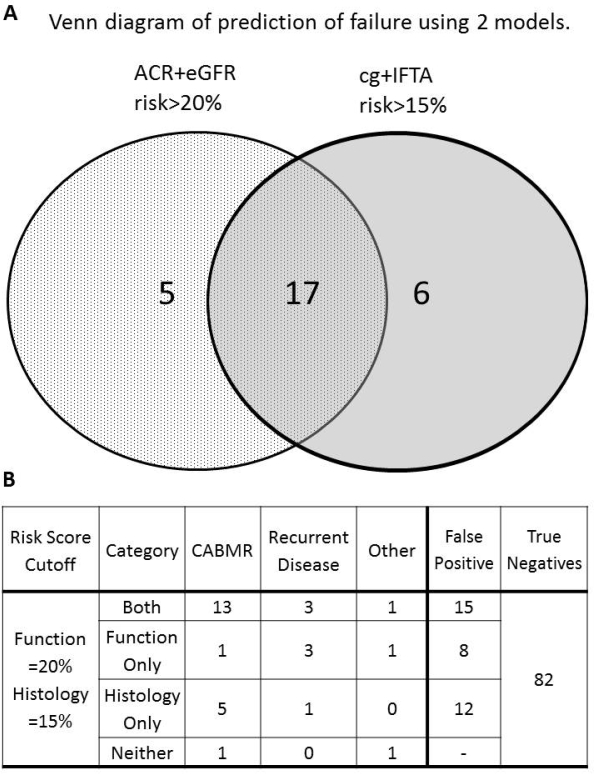
CITATION INFORMATION: Bentall A., Smith B., Park W., Schinstock C., Stegall M. Two Similar Risk Models Identify Different Patients for Graft Loss: Implications for Clinical Trials in DSA+ Kidney Transplant Recipients Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Bentall A, Smith B, Park W, Schinstock C, Stegall M. Two Similar Risk Models Identify Different Patients for Graft Loss: Implications for Clinical Trials in DSA+ Kidney Transplant Recipients [abstract]. https://atcmeetingabstracts.com/abstract/two-similar-risk-models-identify-different-patients-for-graft-loss-implications-for-clinical-trials-in-dsa-kidney-transplant-recipients/. Accessed February 16, 2026.« Back to 2018 American Transplant Congress
