Treatment of Hepatic Encephalopathy in Cirrhotic Patients: Does Rifaximin Prevent Clostridioides Difficile Infection? A Retrospective Analysis
J. D. Goldman, V. Aleksenko, J. L. Davila, N. A. Muhktar, K. Kowdley
Swedish Medical Center, Seattle, WA
Meeting: 2020 American Transplant Congress
Abstract number: B-190
Keywords: Adverse effects, Bacterial infection, Infection, Quality of life
Session Information
Session Name: Poster Session B: All Infections (Excluding Kidney & Viral Hepatitis)
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
*Purpose: Clostridioides difficile infection (CDI) is a major cause of morbidity and mortality and new prevention strategies in high-risk patients are needed. Rifaximin has been assessed for treatment of CDI, but effect on CDI prevention is unknown. We studied patients with cirrhosis who were prescribed rifaximin for hepatic encephalopathy (HE) to determine if prevention of CDI was an off-target benefit.
*Methods: Electronic records were queried from five Seattle-area hospitals. Patients were included in the cohort if diagnosed with cirrhosis or HE and prescribed either lactulose or rifaximin during an index hospitalization. Using proportional hazards regression with competing risk for death, rifaximin exposure through was modeled on incident CDI. Patients were censored on the earliest of CDI, last in-person encounter, or death.
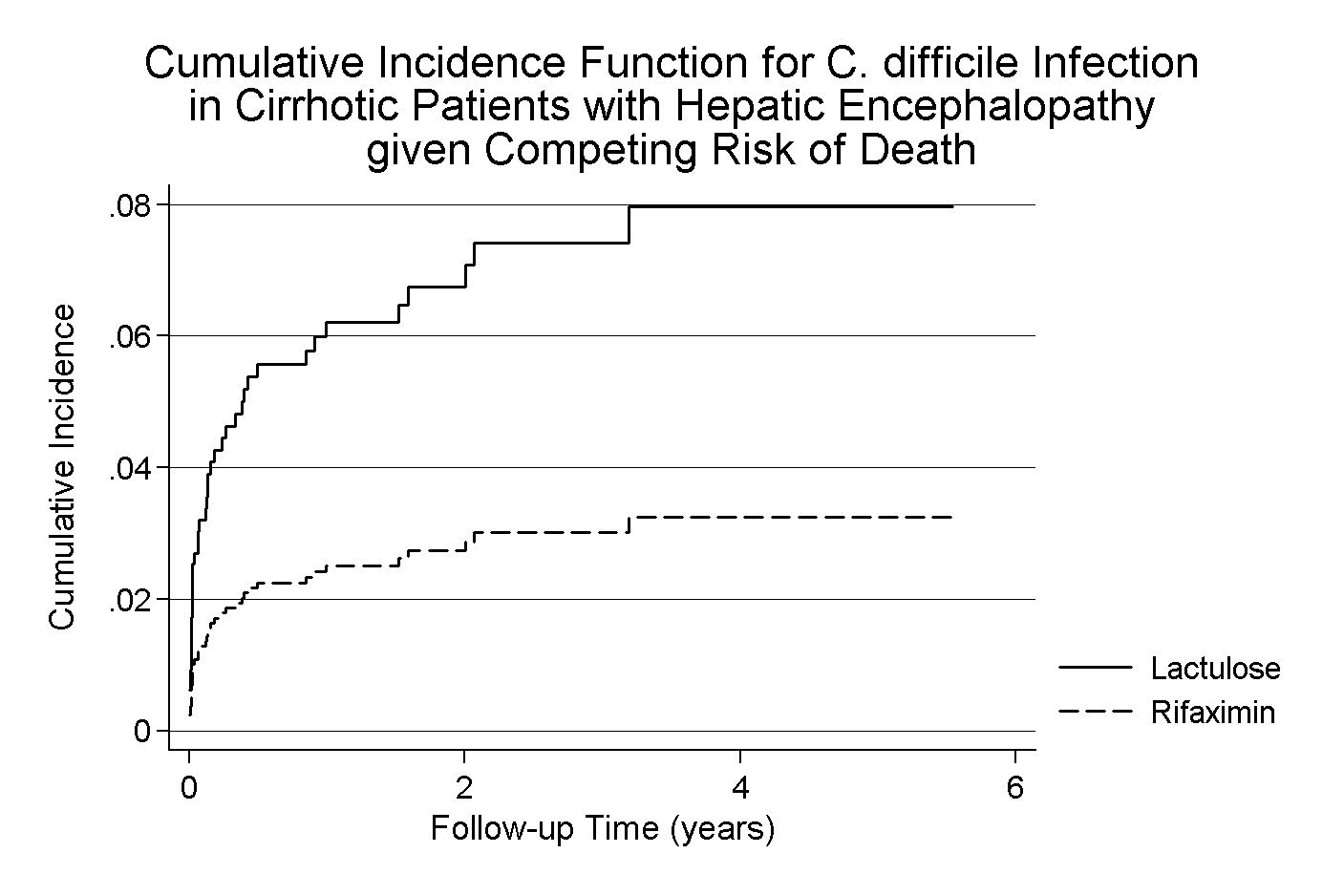
*Results: From 2012 to 2016, 1072 patients with cirrhosis and HE were hospitalized and received treatment for HE. Mean age was 58 years old, 42% were female, and 68% were Caucasian. Patients were followed for a median of 223 (IQR: 36 – 661) days with total cohort follow-up of 1,235 person-years. Nearly all patients received lactulose (96%) and 692 patients (65%) received rifaximin. CDI occurred in 48 patients (incidence 3.9/100 person years), with less CDI in the rifaximin group compared to those who received only lactulose (2.6% vs 7.9%, p < 0.01). Death occurred in 440 patients (41%) with higher mortality in patients exposed to rifaximin and lactulose, compared with lactulose alone (44% vs 35%, p < 0.01), consistent with more advanced liver disease in those receiving dual therapy. Competing with death, the unadjusted sub-hazard ratio (sHR) for CDI was 0.40 (95%CI 0.21 - 0.73), p=0.003 for rifaximin compared with lactulose. Controlling for age, sex, race and MELD on index hospitalization, the sHR was 0.34 (95%CI 0.18 - 0.63), p=0.001.
*Conclusions: Exposure to rifaximin was associated with a reduced risk for CDI in a cohort of patients with cirrhosis and hepatic encephalopathy. Long-term treatment with rifaximin is well-tolerated, and further studies are needed to determine if a reduced risk for CDI can be demonstrated in additional at-risk patient populations.
To cite this abstract in AMA style:
Goldman JD, Aleksenko V, Davila JL, Muhktar NA, Kowdley K. Treatment of Hepatic Encephalopathy in Cirrhotic Patients: Does Rifaximin Prevent Clostridioides Difficile Infection? A Retrospective Analysis [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/treatment-of-hepatic-encephalopathy-in-cirrhotic-patients-does-rifaximin-prevent-clostridioides-difficile-infection-a-retrospective-analysis/. Accessed March 9, 2026.« Back to 2020 American Transplant Congress