Treatment Modality and Outcomes of Antibody-Mediated Rejection in Pediatric Kidney Transplant Recipients: The PARAMOUr Study
1Pediatrics, University of Wisconsin Madison, Madison, WI, 2Wayne State University, Detroit, MI, 3Albert Einstein College of Medicine, New York, NY, 4Augusta University, Augusta, GA, 5The Ohio State University, Columbus, OH, 6University of Minnesota, Minneapolis, MN, 7Emory University, Atlanta, GA, 8University of Utah Health, Salt Lake City, UT, 9The University of Tennessee Health Science Center, Memphis, TN, 10University of Iowa, Iowa City, IA, 11Cedars-Sinai Medical Center, Los Angeles, CA, 12New York Medical College, Valhalla, NY, 13Medical University of South Carolina, Charleston, SC, 14Louisiana State University, New Orleans, LA
Meeting: 2022 American Transplant Congress
Abstract number: 814
Keywords: IVIG, Pediatric, Plasmapheresis, Rejection
Topic: Clinical Science » Kidney » 43 - Kidney: Pediatrics
Session Information
Session Time: 5:30pm-7:00pm
 Presentation Time: 5:30pm-7:00pm
Presentation Time: 5:30pm-7:00pm
Location: Hynes Halls C & D
*Purpose: Antibody-mediated rejection (AMR) is the leading cause of graft failure in kidney transplantation, but evidence for effective treatments is limited.
*Methods: PARAMOUr is a retrospective cohort study of 123 pediatric kidney transplant recipients treated for AMR at 14 Pediatric Nephrology Research Consortium centers 12/31/2009 to 12/31/2019 and followed for one year post-treatment. This study examined the association between treatment regimen and outcome at one year after diagnosis. Effect modification by estimated glomerular filtration rate (eGFR) at diagnosis, donor specific antibody (DSA) class, and pathology was assessed.
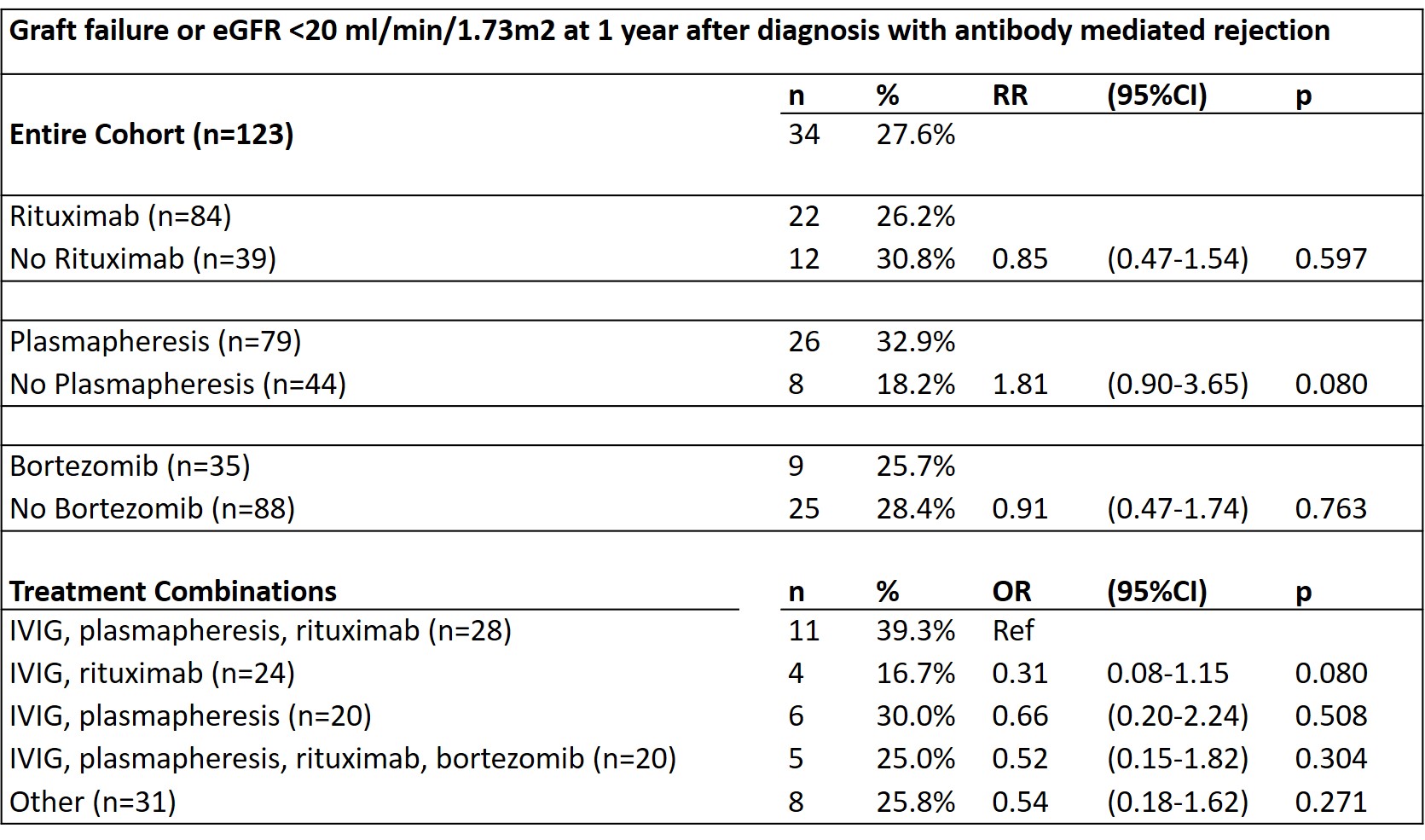
*Results: 88.6% of patients were treated with IVIg, 68.3% with rituximab, 64.2% received plasmapheresis, and 28.5% received bortezomib. 27.6% of patients reached the composite outcome of graft failure or eGFR <20 ml/min/1.73m2 by one year after AMR diagnosis. There were no significant differences in outcome by treatment, whether treatments were analyzed individually or in combination. There was no effect modification by eGFR at diagnosis, predominant DSA class, or pathology findings. Median change in eGFR one year from diagnosis was -2 ml/min/1.73m2 (IQR -11 to 10 ml/min/1.73m2); 37.7% had a decline in eGFR >5 ml/min/1.73m2 while 62.3% had a stable or increased eGFR. An eGFR <30 m/min/1.73m2 at diagnosis was associated with a worse prognosis (RR composite outcome 5.6, IQR 2.9-10.8, p<0.001) but was not associated with treatment choice.
*Conclusions: Specific treatment regimen was not associated with outcome, but 61% of patients did show a stable or improved eGFR after treatment. eGFR at the time of AMR diagnosis was most strongly associated with outcome. Further research on effective treatments for antibody mediated rejection are needed to improve long-term kidney allograft survival.
To cite this abstract in AMA style:
Engen R, Jain A, Hayde N, Mansuri A, Kallash M, McEwen S, Garro R, Peterson C, Zahr R, Harshman L, Puliyanda D, Solomon S, Twombley K, Ashoor I. Treatment Modality and Outcomes of Antibody-Mediated Rejection in Pediatric Kidney Transplant Recipients: The PARAMOUr Study [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/treatment-modality-and-outcomes-of-antibody-mediated-rejection-in-pediatric-kidney-transplant-recipients-the-paramour-study/. Accessed February 26, 2026.« Back to 2022 American Transplant Congress