Tracking the In Vitro Phenotype and Proliferative Capacity of CD57+ CD4 T Cells.
1Duke University, Durham
2Bristol-Meyers Squibb, Princeton
Meeting: 2017 American Transplant Congress
Abstract number: 182
Keywords: CD4, Immunosuppression, Kidney transplantation, T cell activation
Session Information
Session Name: Concurrent Session: Pathways of Clinical Rejection
Session Type: Concurrent Session
Date: Sunday, April 30, 2017
Session Time: 4:30pm-6:00pm
 Presentation Time: 4:54pm-5:06pm
Presentation Time: 4:54pm-5:06pm
Location: E351
CD57+PD1- CD4 T cells are primed effectors with increased expression of adhesion molecules capable of infiltrating allografts and mediating belatacept-resistant rejection (BRR). Whether CD57 is mechanistically requisite or merely a flag for terminally differentiated cells capable of mediating BRR is unknown. Importantly, these cells are virtually non-existent in the healthy population, but significantly increased in patients with renal failure, highlighting the need to study these cells within the patient population. Here, we tracked in vitro biology of CD57+ CD4 T cells from patients with renal failure to gain insights into the ability of cells to proliferate in response to various stimuli. PBMCs were obtained from patients with renal failure and unlabeled or labeled with Violet Proliferation Dye 450 (VPD450), which dilutes out with each cell division. VPD450 labeled cells were sorted into four subsets of CD4 T cells based on expression of CD57 and PD1. Sorted cells were placed in culture with unlabeled bulk PBMCs and either non-stimulated, or stimulated with αCD3, or αCD3 and αCD28. After 4 days cells were removed from culture and assessed for phenotypic changes and proliferation. CD57+PD1- cells remain relatively stable and do not proliferate in unstimulated conditions. When stimulated with plate bound αCD3, the majority of CD57+ cells proliferate for multiple divisions demonstrating their costimulation independence. When stimulated with plate bound αCD3 and soluble αCD28, CD57+ cells exhibit the same proliferative capacity, and CD57- cells exhibit robust proliferation. 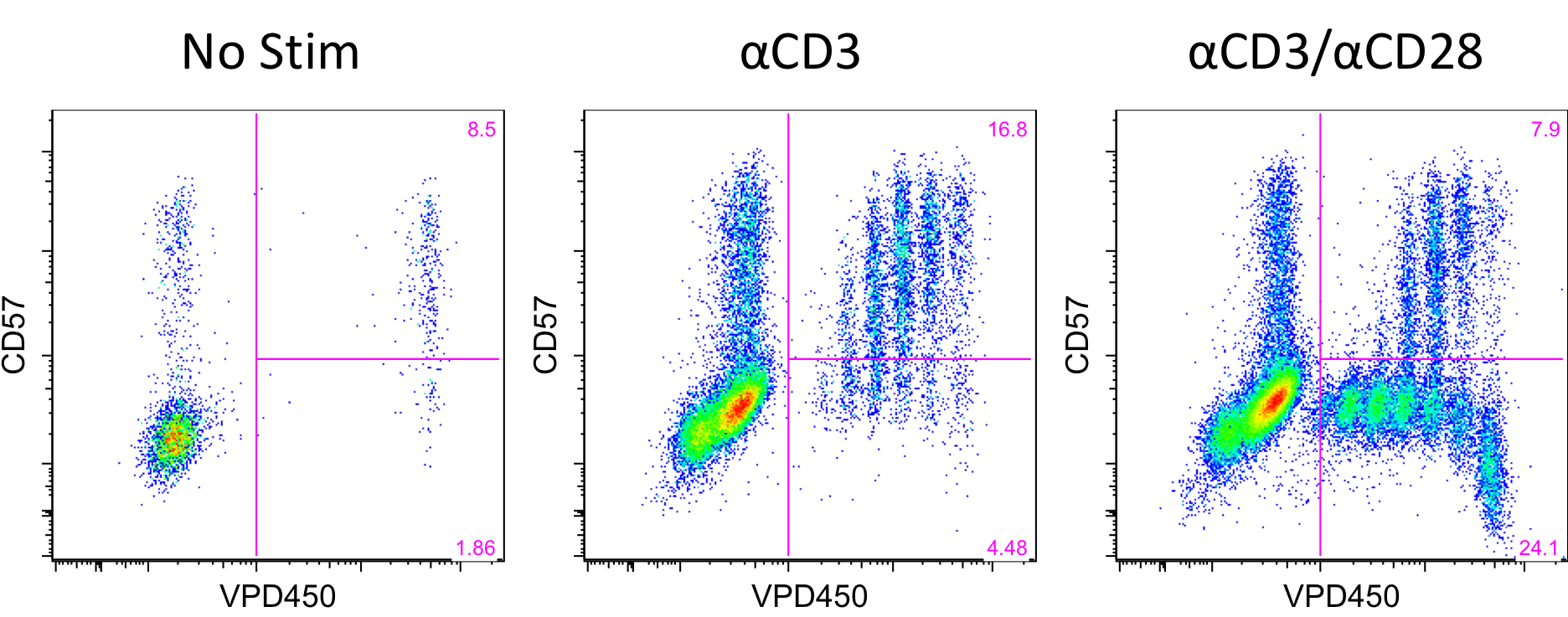 This differential response to stimulation further signifies the unique functionality and requirements of CD57+ vs CD57- CD4 T cells. Patient sample collection is ongoing and future assays include dosing in immunosuppressive agents and allo targets to determine the susceptibility of CD57+ CD4 T cells to suppression with other clinically available therapeutics. This method of tracking PBMCs can be used to segregate costimulation independent and dependent proliferation and identify other immunosuppressive agents to effectively inhibit cellular responses on a patient-by-patient basis.
This differential response to stimulation further signifies the unique functionality and requirements of CD57+ vs CD57- CD4 T cells. Patient sample collection is ongoing and future assays include dosing in immunosuppressive agents and allo targets to determine the susceptibility of CD57+ CD4 T cells to suppression with other clinically available therapeutics. This method of tracking PBMCs can be used to segregate costimulation independent and dependent proliferation and identify other immunosuppressive agents to effectively inhibit cellular responses on a patient-by-patient basis.
CITATION INFORMATION: Espinosa J, Lockley J, Shaw F, Osborne R, Joshi M, Woody T, Ellis M, Hollister B, Cheeseman J, Townsend R, Kirk A. Tracking the In Vitro Phenotype and Proliferative Capacity of CD57+ CD4 T Cells. Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Espinosa J, Lockley J, Shaw F, Osborne R, Joshi M, Woody T, Ellis M, Hollister B, Cheeseman J, Townsend R, Kirk A. Tracking the In Vitro Phenotype and Proliferative Capacity of CD57+ CD4 T Cells. [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/tracking-the-in-vitro-phenotype-and-proliferative-capacity-of-cd57-cd4-t-cells/. Accessed February 22, 2026.« Back to 2017 American Transplant Congress
