Tolerability of De Novo and Conversion Belatacept Regimens in Kidney Transplantation
1Pharmaceutical Services, UCSF Medical Center, San Francisco, CA, 2Transplant Nephrology, UCSF Medical Center, San Francisco, CA
Meeting: 2021 American Transplant Congress
Abstract number: 936
Keywords: Adverse effects, Co-stimulation, Kidney transplantation, Renal function
Topic: Clinical Science » Kidney » Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Information
Session Name: Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Type: Poster Abstract
Session Date & Time: None. Available on demand.
Location: Virtual
*Purpose: Belatacept, a selective T lymphocyte co-stimulation blocker, has been shown to be a safe and effective maintenance therapy for immunosuppression. We investigated the long-term tolerability of belatacept in both de novo and conversion regimens in kidney transplant recipients.
*Methods: This is a retrospective, single-center analysis of all kidney transplant recipients who received belatacept prior to June 30, 2020. We examined the length of time that patients received belatacept, reasons for discontinuation, estimated glomerular filtration rate (eGFR), and incidence of acute rejection.
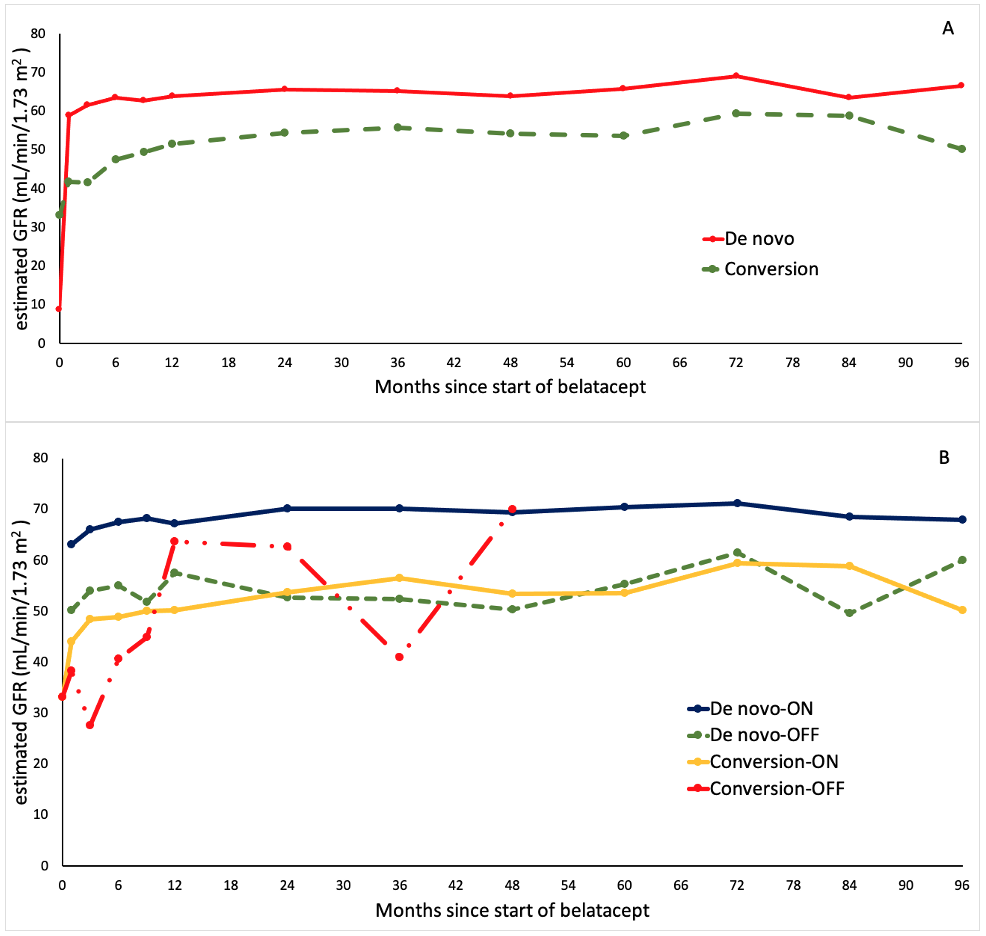
*Results: One-hundred and thirty-six patients were included in the analysis, 91 de novo (67%) and 45 conversion (33%). The median time from transplant to conversion was 4 months (IQR 1.9-6.1, range 0.4-122.9). Eighty-seven patients remained on belatacept, while 42 patients discontinued belatacept for the medical reasons shown in Table 1. There was no statistically significant difference in the rate of discontinuation between de novo and conversion groups (34.1% vs 40%, p = 0.57). In patients who stopped belatacept, the median time from the start of therapy to discontinuation was 3.9 months (IQR 2.2-5.6, range 0.2-151.7) in the de novo group vs 2.5 months (IQR 0.8-4.2, range 0.9-52.5) in the conversion group (p = 0.41). The mean eGFR at 1-year after starting belatacept was significantly higher in the de novo group (63.8 ± 34.4 mL/min/1.73 m2 vs 51.6 ± 16.8 mL/min/1.73 m2, p = 0.02). Figure 1 shows mean eGFR for up to 8 years. The rate of acute rejection at 1-year was similar between de novo and conversion groups (18.7% vs 22.2%, p = 0.65).
*Conclusions: Belatacept was discontinued in a substantial number of patients for various medical reasons. Of these reasons, acute rejection was the most common. The length of time that patients received belatacept was similar between de novo and conversion patients, as were the rates of rejection. Patients started on belatacept de novo maintained a higher eGFR than those who were converted.
$$MISSING OR BAD TABLE SPECIFICATION {3AC34157-FC0F-442A-A79C-4553BF30ADEA}$$
| De novo (n = 26) | Conversion (n = 16) | |
| Acute rejection | 17 (65.4%) | 7 (43.8%) |
| Infection | 2 (7.7%) | 6 (37.5%) |
| Tolerability to regimen | 5 (19.2%) | 3 (18.7%) |
| Others | 2 (7.7%) | – |
Figure 1. Renal function during belatacept regimen. (A) Between de novo and conversion groups. (B) Between subgroups (staying ON belatacept vs switching OFF)
To cite this abstract in AMA style:
Le T, Shoji J, Phillips J, Quan D. Tolerability of De Novo and Conversion Belatacept Regimens in Kidney Transplantation [abstract]. Am J Transplant. 2021; 21 (suppl 3). https://atcmeetingabstracts.com/abstract/tolerability-of-de-novo-and-conversion-belatacept-regimens-in-kidney-transplantation/. Accessed February 19, 2026.« Back to 2021 American Transplant Congress