Time in Therapeutic Range Predicts DSA Development and Acute Rejection in Kidney Recipients with High Tacrolimus Coefficient of Variation
University of Colorado, Aurora, CO.
Meeting: 2018 American Transplant Congress
Abstract number: 435
Keywords: HLA antibodies, Immunosuppression, Risk factors
Session Information
Session Name: Concurrent Session: Kidney Immunosuppression: General Considerations - 1
Session Type: Concurrent Session
Date: Tuesday, June 5, 2018
Session Time: 2:30pm-4:00pm
 Presentation Time: 3:18pm-3:30pm
Presentation Time: 3:18pm-3:30pm
Location: Room 6C
High tacrolimus trough coefficient of variation (TAC CV) has been associated with an increased risk of de novo donor-specific antibody (dnDSA) and acute rejection (AR). We have previously shown lower tacrolimus time in therapeutic range (TAC TTR) also predicts dnDSA and AR. The purpose of this study was to evaluate the risk of dnDSA and AR in patients with a high TAC CV stratified by TAC TTR.
From 2007 to 2013, kidney transplant recipients who were initiated and maintained on tacrolimus in the first year of transplant were screened for dnDSA at months 1, 6, 12 and when clinically indicated. Tacrolimus troughs from 1 week to 12 months were used and troughs after dnDSA or acute rejection were excluded. TAC CV was calculated as (SD/mean)x100%. The Rosendaal method was applied to calculate TAC TTR using a therapeutic range of 5 – 10 ng/ml. AR included clinical cellular and/or antibody-mediated rejection. Logistic regression was used to calculate odds ratios and Cox's proportional hazards regression for hazard ratios for time to AR, adjusted for HLA mismatches, age, ethnicity, donor type, gender, prior transplant, induction therapy, MMF dose reduction and delayed graft function.
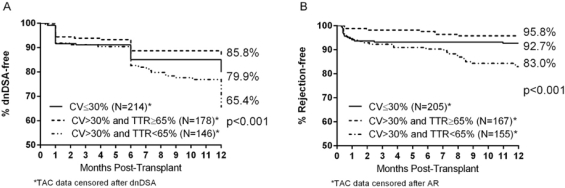
Of 538 patients included in the analysis, 117 (21.7%) developed dnDSA and 53 (9.9%) developed AR within 12 months of transplant. 324 (60%) of patients had a TAC CV >30%. Of these, 178 (55%) had a TAC TTR ≥65% and 146 (45%) had a TAC TTR <65%. In those patients with a high (>30%) TAC CV, TAC TTR <65% was associated with a significant risk of dnDSA development in multivariable analysis (odds ratio 3.59, 95% CI 1.97-6.56, p<0.001) compared to a TAC TTR ≥65%. Similarly, in patients with TAC CV >30% the risk of AR at 1 year was significantly increased for those with TAC TTR <65% vs. ≥65% (hazard ratio 4.66, 95% CI 1.99-10.89, p<0.001). Kaplan-Meier estimates of dnDSA and AR in patients by TAC CV and TAC TTR are shown in figure 1.
These data suggest previously reported risk of immunologic injury associated with higher tacrolimus trough variability is largely due to time outside of therapeutic range as opposed to the variability itself.
CITATION INFORMATION: Davis S., Gralla J., Klem P., Wiseman A., Cooper J. Time in Therapeutic Range Predicts DSA Development and Acute Rejection in Kidney Recipients with High Tacrolimus Coefficient of Variation Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Davis S, Gralla J, Klem P, Wiseman A, Cooper J. Time in Therapeutic Range Predicts DSA Development and Acute Rejection in Kidney Recipients with High Tacrolimus Coefficient of Variation [abstract]. https://atcmeetingabstracts.com/abstract/time-in-therapeutic-range-predicts-dsa-development-and-acute-rejection-in-kidney-recipients-with-high-tacrolimus-coefficient-of-variation/. Accessed February 22, 2026.« Back to 2018 American Transplant Congress