Thymoglobulin Versus IL2RA: Three Year Results of a Randomized Controlled Trial
Transplant, MUSC, Charleston, SC.
Meeting: 2015 American Transplant Congress
Abstract number: 283
Keywords: African-American, Induction therapy, Rejection
Session Information
Session Name: Concurrent Session: Kidney: Induction
Session Type: Concurrent Session
Date: Monday, May 4, 2015
Session Time: 4:00pm-5:30pm
 Presentation Time: 4:00pm-4:12pm
Presentation Time: 4:00pm-4:12pm
Location: Room 120-ABC
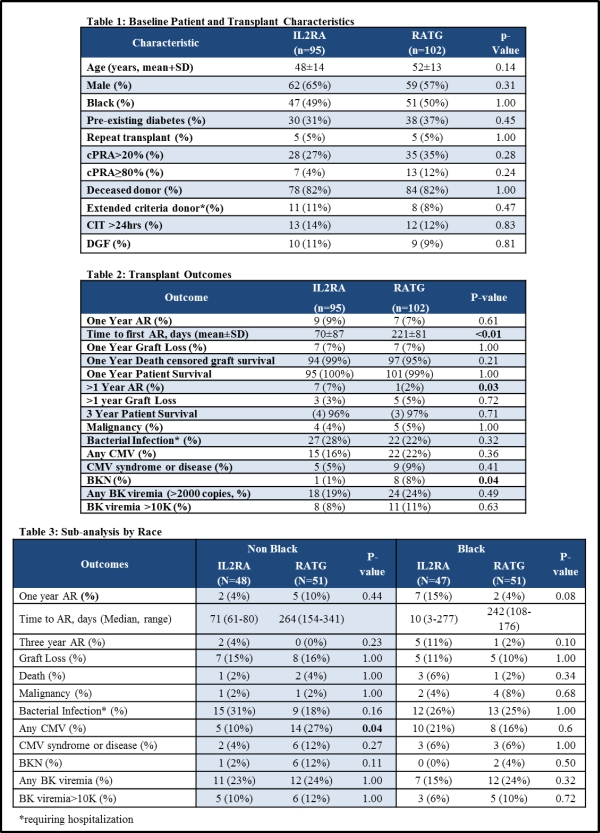
This is a three year follow-up of a prospective, randomized controlled trial (RCT) to determine the safety and efficacy of induction with rabbit antithymocyte globulin (rATG) compared to IL2RA in a moderate to high risk transplant population under modern immunosuppression.
Methods: Three-year follow-up of a single-center, open-labeled, RCT at a large, racially diverse, transplant center. Patients were randomized to receive IL2RA versus rATG for induction in combination with tacrolimus, MMF and corticosteroids. Patients were risk-stratified to ensure equality between groups for race, repeat transplant, PRA>20% and CIT>24hrs.
Results: 200 patients (n=98 in the IL2RA group and n=102 in the rATG group) were enrolled (3 patients in the IL2RA group were excluded in the 3 year analysis following receipt of a pancreas transplant). Baseline demographics were the same in each group (Table 1). BPAR rates after 1-year were significantly lower in the rATG group compared to the IL2RA group (2% vs.7%, p=0.03, Table 2). Rejections occurring after 1 year resulted predominantly following non-adherence or sub-therapeutic immunosuppression. At 3 years, the incidence of BKN was higher in the rATG group (8% vs. 1%, p=0.04). Subgroup analysis based on race demonstrated no differences in infections with a strong trend towards lower rejection rates in black patients in the rATG group; whereas non-black patients experienced a higher incidence in bacterial infections requiring hospitalization in the IL2RA group and more CMV infections in the rATG group [Table 3].
Conclusions: rATG induction provides improved protection against early and late acute rejection, predominantly in black renal transplant recipients, while IL2RA yielded an increased risk of bacterial infections in non-black recipients.
To cite this abstract in AMA style:
Pilch N, Meadows H, Taber D, Fleming J, Mardis C, Nadig S, McGillicuddy J, Srinivas T, Bratton C, Baliga P, Chavin K. Thymoglobulin Versus IL2RA: Three Year Results of a Randomized Controlled Trial [abstract]. Am J Transplant. 2015; 15 (suppl 3). https://atcmeetingabstracts.com/abstract/thymoglobulin-versus-il2ra-three-year-results-of-a-randomized-controlled-trial/. Accessed February 19, 2026.« Back to 2015 American Transplant Congress
