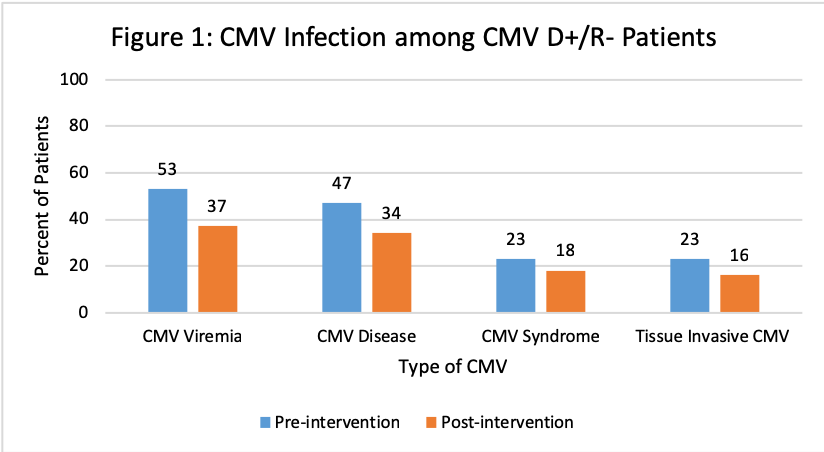
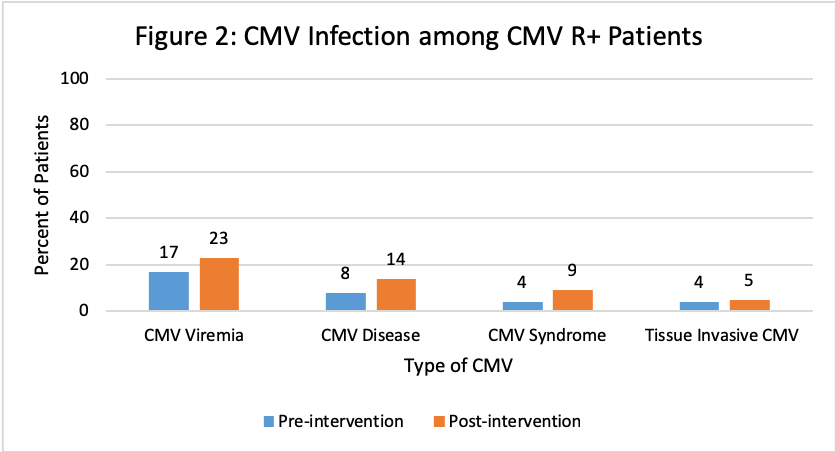
The Value of Pharmacist-Driven Valganciclovir Dosing in Kidney Transplant Recipients in Reducing CMV Infection: A Single Center Experience
K. D. Belfield1, C. E. Costello2, M. Malinis3
1Department of Pharmacy, Yale New Haven Hospital, New Haven, CT, 2University of Connecticut School of Pharmacy, Storrs, CT, 3Section of Infectious Diseases,, Yale School of Medicine, New Haven, CT
Meeting: 2020 American Transplant Congress
Abstract number: A-203
Keywords: Cytomeglovirus
Session Information
Session Name: Poster Session A: Kidney Infectious Excluding Polyoma & Viral Hepatitis
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
*Purpose: Cytomegalovirus (CMV) is a common viral infection after kidney transplant (KT) despite prophylaxis with valganciclovir (VGCV). VGCV is renally dosed and can be a challenge among KT recipients (KTR) whose renal function can fluctuate post-transplant. We previously did a review of our data and noted frequent inappropriate VGCV dosing and high rates of CMV infection. Since 2017, our center eliminated VGCV half dosing in CMV R+ KTR and employed pharmacist monitoring of VGCV dosing. The objective of this study was to evaluate the efficacy of both interventions in pre- and post-intervention cohorts.
*Methods: A retrospective chart review was conducted of adult KTR who underwent KT between 3/2013-2/2015 pre-intervention and 6/2017-12/2018 post-intervention. KTR with multiorgan transplant or who died within the study period were excluded. The primary outcome was rate of CMV disease in CMV D+/R- and CMV R+ patients. Secondary outcomes included rate of overall CMV infection and appropriateness of VGCV dosing. Appropriateness of VGCV dosing was examined at hospital discharge and monthly for the duration of prophylaxis.
*Results: A total of 131 and 150 KTR were included in the pre- and post-intervention groups, respectively. Patients were similar at baseline with significantly more anti-thymoctye globulin induction and deceased donors pre-intervention and significantly more males, alemtuzumab induction and living donors post-intervention. Overall rates of CMV disease were similar, however there was a decrease in the CMV D+/R- patients. Improvement in appropriateness of VGCV dosing between groups was observed in CMV D+/R- patients at all time points and at discharge for CMV R+.
*Conclusions: Pharmacist monitoring of VGCV dosing after KT resulted in improved appropriateness of dosing, particularly in CMV D+/R-. Rates of CMV infection were similar among CMV R+, but the occurrence of CMV disease was lower in CMV D+/R- KTR post-intervention. This study demonstrated the value of transplant pharmacists in the implementation of a CMV prevention strategy in KTR.
To cite this abstract in AMA style:
Belfield KD, Costello CE, Malinis M. The Value of Pharmacist-Driven Valganciclovir Dosing in Kidney Transplant Recipients in Reducing CMV Infection: A Single Center Experience [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/the-value-of-pharmacist-driven-valganciclovir-dosing-in-kidney-transplant-recipients-in-reducing-cmv-infection-a-single-center-experience/. Accessed February 27, 2026.« Back to 2020 American Transplant Congress